Consider a system of N free electrons within a volume V. Even at absolute zero, such a system exerts a pressure P on its surroundings due to the motion of the electrons. To calculate this pressure, imagine that the volume increases by a small amount dV. The electrons will do an amount of work PdV on their surroundings, which means that the total energy Etot of the electrons will change by an amount dEtot = -PdV. Hence P = -dErot/dV. (a) First, write down the expression of Etot as an integral involving the density of states g(E) and the Fermi- Dirac distribution function f(E) at temperature T. Then evaluate this integral by setting T = 0, and obtain Etot as a function of N and the Fermi energy EF0- (b) Show that the pressure of the electrons at absolute zero is 2 N P = EFo- 5 V (c) Calculate EFo and P for solid copper that has a free-electron concentration of 8.45 x 1028 m-3. Express EFo and P in electronvolts and atmospheres, respectively. (d) The pressure you found in part (c) is extremely high. Why, then, don't the electrons in a piece of copper simply explode out of the metal?
Consider a system of N free electrons within a volume V. Even at absolute zero, such a system exerts a pressure P on its surroundings due to the motion of the electrons. To calculate this pressure, imagine that the volume increases by a small amount dV. The electrons will do an amount of work PdV on their surroundings, which means that the total energy Etot of the electrons will change by an amount dEtot = -PdV. Hence P = -dErot/dV. (a) First, write down the expression of Etot as an integral involving the density of states g(E) and the Fermi- Dirac distribution function f(E) at temperature T. Then evaluate this integral by setting T = 0, and obtain Etot as a function of N and the Fermi energy EF0- (b) Show that the pressure of the electrons at absolute zero is 2 N P = EFo- 5 V (c) Calculate EFo and P for solid copper that has a free-electron concentration of 8.45 x 1028 m-3. Express EFo and P in electronvolts and atmospheres, respectively. (d) The pressure you found in part (c) is extremely high. Why, then, don't the electrons in a piece of copper simply explode out of the metal?
Related questions
Question
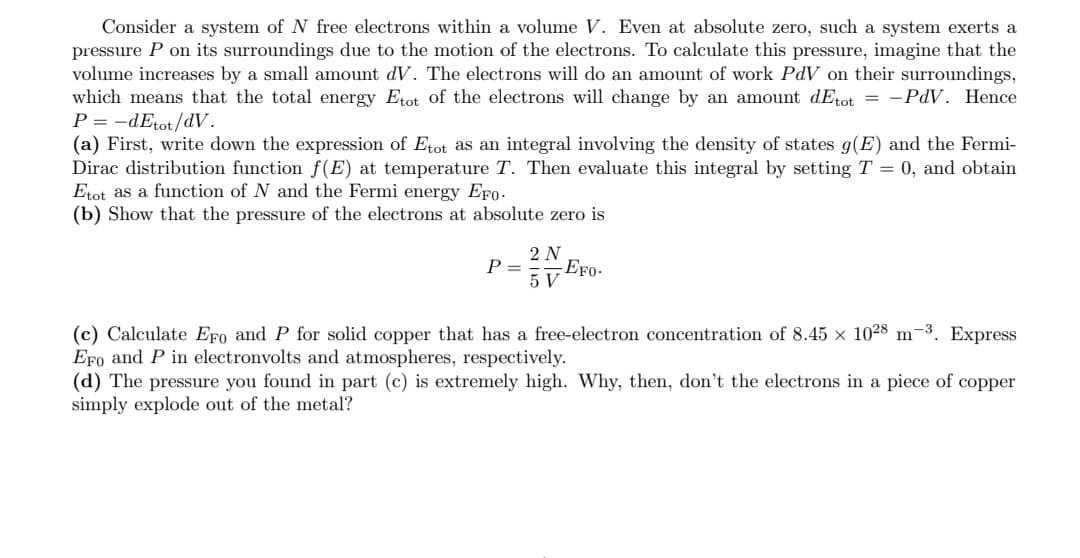
Transcribed Image Text:Consider a system of N free electrons within a volume V. Even at absolute zero, such a system exerts a
pressure P on its surroundings due to the motion of the electrons. To calculate this pressure, imagine that the
volume increases by a small amount dV. The electrons will do an amount of work PdV on their surroundings,
which means that the total energy Etot of the electrons will change by an amount dEtot = -PdV. Hence
P = -dEtot/dV.
(a) First, write down the expression of Etot as an integral involving the density of states g(E) and the Fermi-
Dirac distribution function f(E) at temperature T. Then evaluate this integral by setting T = 0, and obtain
Etot as a function of N and the Fermi energy EFo.
(b) Show that the pressure of the electrons at absolute zero is
2 N
P =
EFo-
5 V
(c) Calculate EFo and P for solid copper that has a free-electron concentration of 8.45 × 1028 m-3. Express
EFo and P in electronvolts and atmospheres, respectively.
(d) The pressure you found in part (c) is extremely high. Why, then, don't the electrons in a piece of copper
simply explode out of the metal?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 10 images
