Consider the following two steps process. Heat is allowed to flow out of an ideal gas at constant volume so that it's pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9L to 9.34L, where the temperature reaches it's original value as shown below calculate, a) the total work done by the gas in the process. b) the Change in internal energy of the gas in the process and c) the total heat flow into and out of the gas
Consider the following two steps process. Heat is allowed to flow out of an ideal gas at constant volume so that it's pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9L to 9.34L, where the temperature reaches it's original value as shown below calculate, a) the total work done by the gas in the process. b) the Change in internal energy of the gas in the process and c) the total heat flow into and out of the gas
Related questions
Question
Consider the following two steps process. Heat is allowed to flow out of an ideal gas at constant volume so that it's pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9L to 9.34L, where the temperature reaches it's original value as shown below calculate, a) the total work done by the gas in the process.
b) the Change in internal energy of the gas in the process and
c) the total heat flow into and out of the gas
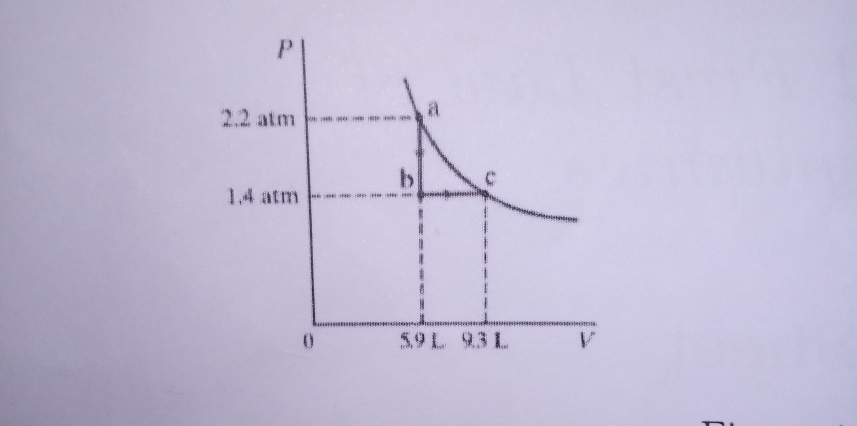
Transcribed Image Text:a
2.2 atm
m nm meni e me
1.4 atm
59L 93L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
