Considering the concepts besides this problem, identify the thermodynamic process that occurs in the system using the PV diagram of an argon below. Answer: Adiabatic 6.0 Pa- 2.0 Pa 0.01 m³ 0.04 m³ Adiabatic Process Recall that an adiabatic process is characterized by zero net heat flow into or out of a system (Q=0). Heat flow can be prevented by either surrounding the system with thermally insulating material or by carrying out the process so quickly that there is negligible time for heat flow to happen. The PV diagram of an adiabatic process is shown in Figure 5. Notice how the system crosses two isotherms. This indicates that temperature changes may occur during an adiabatic process, aside from changing pressure and volume of the system. In the case shown in figure 5, the system expands as it cools down adiabatically. P1 1 (P₂. V₂) W (P₂- V₂) V (m³)
Considering the concepts besides this problem, identify the thermodynamic process that occurs in the system using the PV diagram of an argon below. Answer: Adiabatic 6.0 Pa- 2.0 Pa 0.01 m³ 0.04 m³ Adiabatic Process Recall that an adiabatic process is characterized by zero net heat flow into or out of a system (Q=0). Heat flow can be prevented by either surrounding the system with thermally insulating material or by carrying out the process so quickly that there is negligible time for heat flow to happen. The PV diagram of an adiabatic process is shown in Figure 5. Notice how the system crosses two isotherms. This indicates that temperature changes may occur during an adiabatic process, aside from changing pressure and volume of the system. In the case shown in figure 5, the system expands as it cools down adiabatically. P1 1 (P₂. V₂) W (P₂- V₂) V (m³)
Related questions
Question
Please explain detailedly if possible explain with the use of the graph:
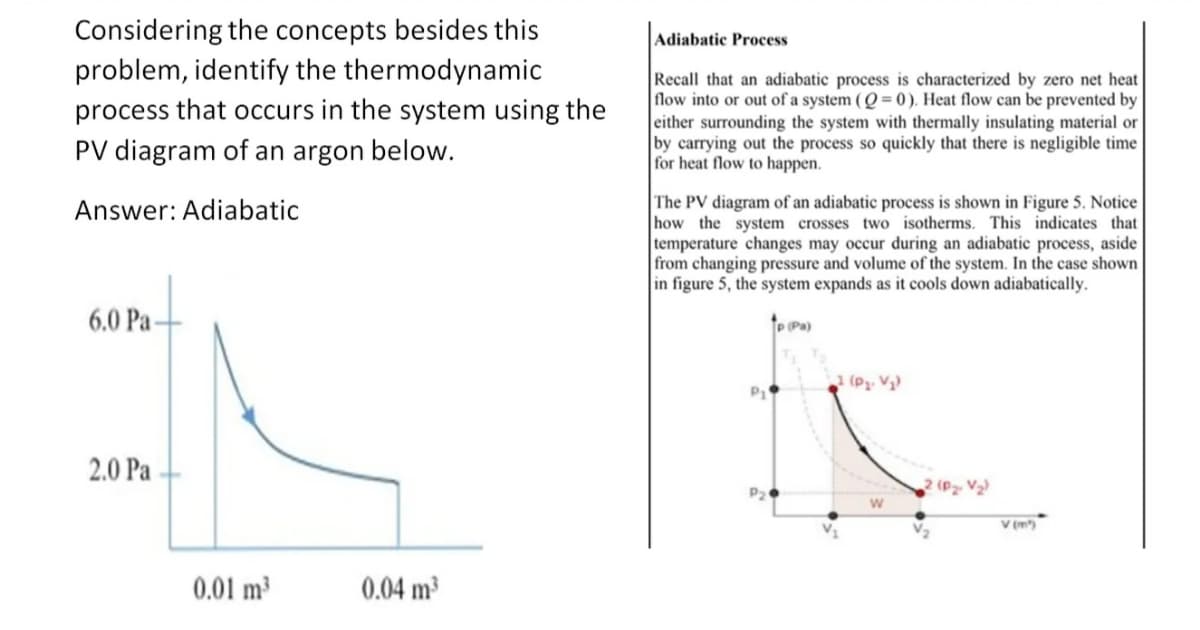
Transcribed Image Text:Considering the concepts besides this
problem, identify the thermodynamic
process that occurs in the system using the
PV diagram of an argon below.
Answer: Adiabatic
6.0 Pa-
2.0 Pa
0.01 m³
0.04 m³
Adiabatic Process
Recall that an adiabatic process is characterized by zero net heat
flow into or out of a system (Q=0). Heat flow can be prevented by
either surrounding the system with thermally insulating material or
by carrying out the process so quickly that there is negligible time
for heat flow to happen.
The PV diagram of an adiabatic process is shown in Figure 5. Notice
how the system crosses two isotherms. This indicates that
temperature changes may occur during an adiabatic process, aside
from changing pressure and volume of the system. In the case shown
in figure 5, the system expands as it cools down adiabatically.
P (Pa)
1 (P₂-V₁)
W
? (P₂-V₂)
V (m¹)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
