constant value mass of Imol volume of 1mol gas (100kPa and 300K) Thermal conductivity (100kPa and 300K) Volume of 1mol liquid (100kPa and boiling T) V=28.5cm³. т%39.9g V, =0.0242m3 k =0.0177 Wm-'K-1 And Cy 1 1 ki = lvrms V, 2 4ar2 NA use the thermal conductivity to calculate the size of atoms.(r is the radius of molecules -spheres). Cy/Vg Urms Compute constants like R and k) and obtain a numerical estimate of mean free path and using only the given numbers (so you can't use any number or physical
constant value mass of Imol volume of 1mol gas (100kPa and 300K) Thermal conductivity (100kPa and 300K) Volume of 1mol liquid (100kPa and boiling T) V=28.5cm³. т%39.9g V, =0.0242m3 k =0.0177 Wm-'K-1 And Cy 1 1 ki = lvrms V, 2 4ar2 NA use the thermal conductivity to calculate the size of atoms.(r is the radius of molecules -spheres). Cy/Vg Urms Compute constants like R and k) and obtain a numerical estimate of mean free path and using only the given numbers (so you can't use any number or physical
Related questions
Question
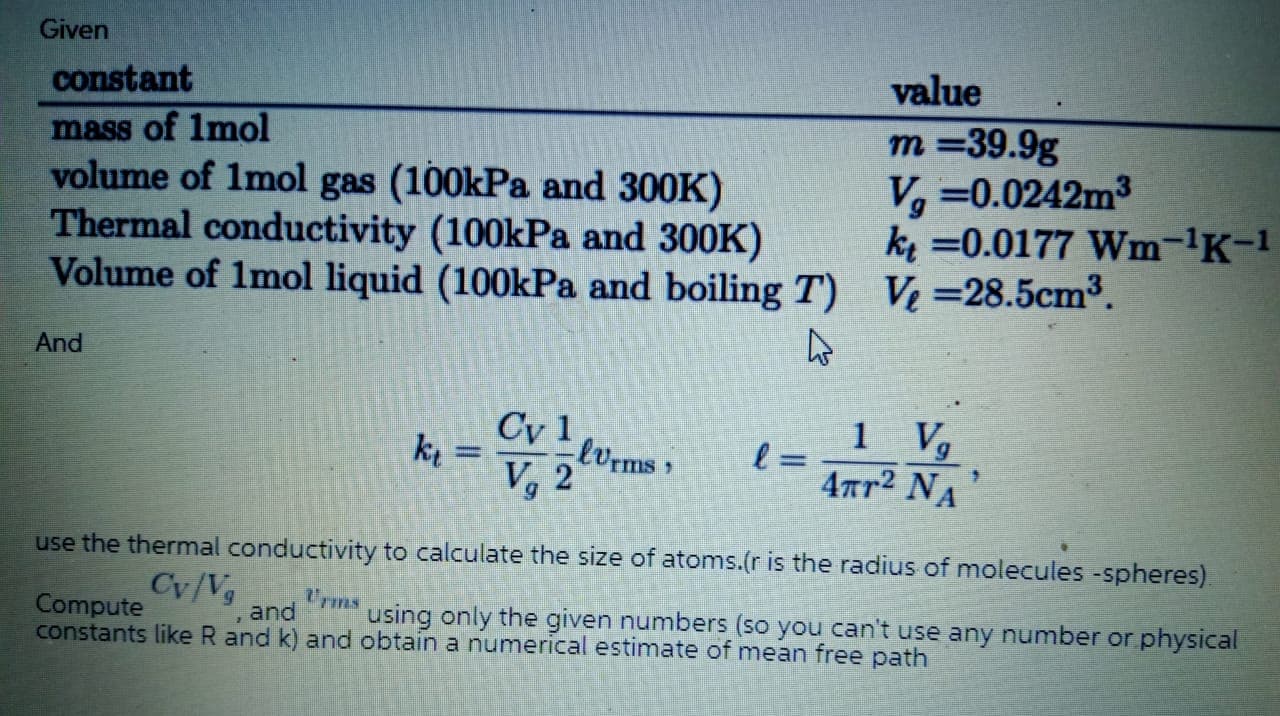
Transcribed Image Text:constant
value
mass of Imol
volume of 1mol gas (100kPa and 300K)
Thermal conductivity (100kPa and 300K)
Volume of 1mol liquid (100kPa and boiling T) V=28.5cm³.
т%39.9g
V, =0.0242m3
k =0.0177 Wm-'K-1
And
Cy 1
1
ki =
lvrms
V, 2
4ar2 NA
use the thermal conductivity to calculate the size of atoms.(r is the radius of molecules -spheres).
Cy/Vg
Urms
Compute
constants like R and k) and obtain a numerical estimate of mean free path
and
using only the given numbers (so you can't use any number or physical
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images
