der these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next Lo the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species relative pH of 0.1 M aqueous solution ☑ ك + C6H5NH3 2 CH3NH2 (Choose one) CIO2 (Choose one) CH,NH, (Choose one) OH CH3NH3 H₂O + 8 (highest) 3 (Choose one) HCIO2 1 (lowest)
der these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next Lo the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. species relative pH of 0.1 M aqueous solution ☑ ك + C6H5NH3 2 CH3NH2 (Choose one) CIO2 (Choose one) CH,NH, (Choose one) OH CH3NH3 H₂O + 8 (highest) 3 (Choose one) HCIO2 1 (lowest)
Chapter3: Molecules Of Life
Section: Chapter Questions
Problem 1SA: Organic molecules consist mainly of _______ atoms. a. carbon c. carbon and hydrogen b. carbon and...
Related questions
Question
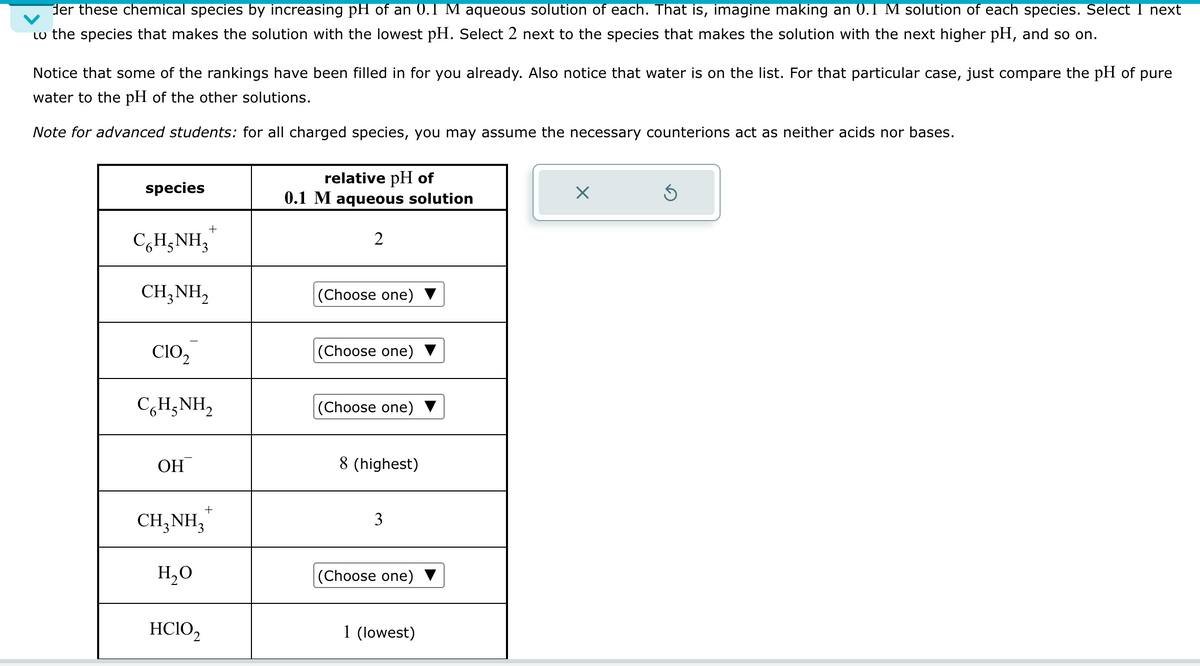
Transcribed Image Text:der these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next
Lo the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on.
Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure
water to the pH of the other solutions.
Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases.
species
relative pH of
0.1 M aqueous solution
☑
ك
+
C6H5NH3
2
CH3NH2
(Choose one)
CIO2
(Choose one)
CH,NH,
(Choose one)
OH
CH3NH3
H₂O
+
8 (highest)
3
(Choose one)
HCIO2
1 (lowest)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning


Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781305073951
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Human Biology (MindTap Course List)
Biology
ISBN:
9781305112100
Author:
Cecie Starr, Beverly McMillan
Publisher:
Cengage Learning