Determine the nuclear magnetic moment of Cl nucleus, using the Shell model. (for a proton g, =1, g, = 5.586 and for a neutron g, = 0, g, =-3.826) (а) 4.79ду (b) 3.79ду (с) 0 (d) 1.91µy
Q: The diameter of an atomic nucleus is about 10 fm (1 fm = 10-15 m). What is the kinetic energy, in Me...
A: E=0.5mv2=0.5mhmλ2=0.5h2mλ2
Q: A ball is thrown upward at 12 m/s from the top of a 50 meter tall building.. (a) What was its final ...
A: (a) We assume that there is no air resistance acting on the ball. Initially the ball is thrown from ...
Q: Each part shows one or more point charges. The charges have equal magnitudes. If a positive charge ...
A: Given: The figures are as follows: The charges have equal magnitudes. Introduction: Electric potent...
Q: If a single constant force acts on an object that moves on a straight line, the object's velocity is...
A: Given: If a single constant force acts on an object that moves on a straight line, the object's velo...
Q: What are the expected readings of the following in the figure below? (R = 5.60 ohms, DeltaV = 5.00 V...
A:
Q: Electrons in an x-ray tube are accelerated to 0.65c. The beam then travels 1.0 cm to a target. What ...
A:
Q: Singly ionized helium has a single orbiting electron, so the mathematicsof the Bohr hydrogen atom wi...
A: Given value, The longest wavelength in hydrogen Balmer series correspond to n=3 to n=2. Energy level...
Q: A researcher has placed a sample of radioactive material in an enclosure and blocked all emissions e...
A: The expression for the magnetic force is as follows: F→=qv→×B→ 1
Q: Consider a uniform solid hemisphere of radius r and mass m which is kept in contact with a smooth wa...
A: Given: The uniform solid hemisphere has mass m. The radius of the uniform solid hemisphere is r. Int...
Q: The graph given below shows the instability of wave through beam of electrons. Can this beam cause L...
A: Landau damping : The damping of a collective mode of oscillations in plasmas without collisions of c...
Q: Objects with masses n m, = 15.0 kg and m, = 8.0 kg are connected by a light string that passes oyér ...
A: Given m1=15.0kg m2=8.0kg Starting from rest m2 falls d=1.00m in time τ=1.23s.
Q: Please help solve #5
A: For the vertical motion of an object, the kinematic equation of motion is given by Where vy = fin...
Q: Show your all calculation
A: Benefits of the programmatic visualization tools: 1) have greater flexibility 2) simple to use ...
Q: A box of mass 5.4 kg is given an initial speed of v0 = 7.1 m/s along a ramp as indicated in the figu...
A:
Q: Using the data given, please answer the question in photo 1.
A: Given Question: In what direction is your friend from the starting point after skiing 2 km east and...
Q: suppose I have a square of charge with a uniform charge density lying in the x-y plane with one corn...
A: Let the area of the given square of charge be denoted by A. Its side length is L as given. It has a ...
Q: The phase space trajectory of an undamped oscillator is shown below. In the diagram, eachdivision al...
A: The maximum velocity is the velocity at the equilibrium position and the Amplitude of the oscillator...
Q: 8. Waves in a Tube Standing waves in a violin string must have nodes at the ends of the string becau...
A: (a) location of the nodes y(x,t)=00.3cosx2cos50πt=0cosx2=0x2=n-12π n≥1x=2n-1π
Q: An infinite grounded conducting plane is located at z = 0 and fills the x – y plane so that the elec...
A: The first four parts have been solved due to the length of the question and the paucity of time. (a)...
Q: You can treat the pottery wheel as a uniform disk and the blob of clay as a point particle. The mome...
A: Given Pottery wheel of radius R =20cm=0.20m And mass M Rotating with angular speed ωi=8π rad/s A blo...
Q: What is the total potential difference between the plates? What is the surface charge densities of t...
A: Using work-energy theorem The sum of the change in kinetic energy of the particle and change in pote...
Q: Lagran equation sprin a mass m by a pulley(disk) of radius a as shown in the figure is given by: 2g ...
A: Since mass of the pulley is not given so we assume it as massless. Since mass and spring are connect...
Q: Occupational safety experts have developed an alternative criterion for electrical safety. They have...
A: A human body’s internal electrical resistance from one hand to the other can be taken to be of aroun...
Q: TOPIC: HELMHOLTZ COILS IN JJ THOMSON'S EXPERIMENT FOR THE ELECTRON MASS LOAD RELATIONSHIP Solve...
A: Given, Helmholtz coil
Q: How can I get the solutions and answer to this problem? Determine the total pressure in psi (pound p...
A: We know, Pressure in a given depth is P=ρgh where ρ is density h is the depth. 1/4 th of the cylind...
Q: Physics word problem on energy/work/force:
A: As per guidelines, the first three sub-parts have been answered. (a) Potential energy of mass m is, ...
Q: 21. A car starts at a green light and accelerates at 6 m/s2. A truck passes through the same light a...
A: The kinematic equation of motion is given by Where u = Initial speed, a = acceleration, t = time, S...
Q: Six parallel-plate capacitors of identical plate separation have different plate areas A, different ...
A: Charge stored in a capacitor Q=CV=εAdV
Q: A firecracker explodes at x = 0 m, t = 0 μs. A second explodes at x = 300 m, t = 2.0 μs. What is the...
A: The expression for the velocity is as follows: v=ΔxΔtv=300 m-0 m2 μs-0 μsv=1.5×108m/s ...
Q: Please solve it
A: 1. a) Given: Semiconductor slabs of equal dimensions of three different materials (Si, Ge, GaAs) are...
Q: Attached is a picture of an object’s position versus time plot. Based on the picture, Determine the...
A:
Q: Please answer the question to the right by using the info on the left. Need to find the maximum heig...
A: Given that the rocket has only fuel to burn for 3 s. Therefore the height covered in 3 s be calculat...
Q: In an image intensification system, trace the information and its carrier from the x ray beam emergi...
A: Introduction: Image intensifiers increase the brightness of the fluoroscopic image and permit the ob...
Q: Consider the metal plate in Fig. 2 being held in place by two cables AC and AB attached to a common ...
A: Solving only 3 parts due to time boundation.
Q: A particle accelerator is three kilometers long and accelerates electrons ( mass of electron = 9.11 ...
A: Erest=m0c2
Q: Advanced Physics Question
A: Let q1, q2 denote the charges, r denote the distance, k denote the Coulomb constant, U denote the ch...
Q: Consider a metal sphere falling through a liquid such as water. Which of the following spheres will...
A: The forces which are acting on an object falling in water are gravitational force downwards and drag...
Q: Six parallel-plate capacitors of identical plate separation have different plate areas A, different ...
A: The capacitance of the parallel plate capacitor is given by Where k = Dielectric constant, A = Area...
Q: A large tank open to the atmosphere is filled with water to a height of 5 m from the outlet tap . A ...
A: Using Bernoulli's theorem P1+12ρV12+ρgz1=P2+12ρV22+ρgz2ρgz1=12ρV22 P1=P2;z2=0;V1=0V2=...
Q: 03
A: Moment of inertia is along Ix' = Ix cos θ i,e 1.26x106 mm4 32= 1.0911 x106 mm4 Iy' = Iy cos 3...
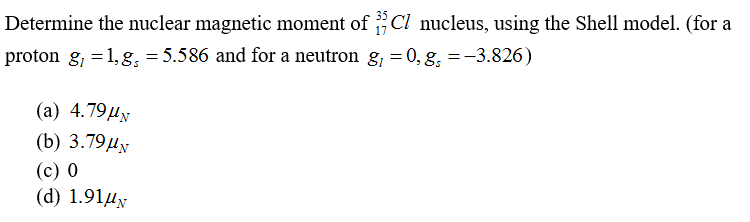
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
