Determine the nuclear magnetic moment of Cl nucleus, using the Shell model. (for a proton g, = 1, g: = 5.586 and for a neutron g, = 0, g, =-3.826) %3D
Q: A 60 kg package is falling downward with an acceleration of 3.00 m/s² when its velocity is 30.0 m/s....
A:
Q: Find the displacement for Spring mass damper system, m=1, c=2, k=5, All units are standard. initial ...
A:
Q: 7. A horizontal pipe with circular cross-section delivers 4 kg of water per second. The diameter of ...
A: Initial velocity m˙1=ρA1v1v1=m˙1ρA1
Q: At a basketball match, a player used his head to (unintentionally) deflect a ball straight up, and 0...
A: Given: When the ball was in air Time (T) = 0.5 seconds. g = 10 ms2
Q: The wavefunction of the particle in its ground state is (x)=CePH. The first order correction to the ...
A:
Q: Let f(ɛ) be the Fermi-Dirac distribution function and u be the chemical potential. Obtain the expres...
A: Solution: The Fermi-Dirac distribution function is given as Where
Q: A 10 g rubber ball and a 10 g clay ball are thrown at a wall with equal speeds. The rubber ball bou...
A: given mass of the rubber ball (mb) and clay ball(mc) is mb=mc=m=10g the initial speed of both the ba...
Q: figure shows an downhill plane with the dimensions as H = 15 m, D = 19 m, and an angle ? = 54.14o. I...
A:
Q: Calculate the magnetic flux density for the current distribution in free space to be A = (3x²y+ yz )...
A: Given: The vector potential for the current distribution in the free space is given as
Q: In the figure above, ?1=6.9??, ?2=4.6??. Find the equivalent capacitance of the circuit in units of ...
A:
Q: Explain briefly the effect of different types of controllers (P, I and PD) on the step responseand t...
A: Comparison of step responses Fig 1: Comparison of PID Controller on step response
Q: Find the current in the switch at the same time. The current through the conductor at this time is 1...
A:
Q: A door has a height of 2.1 m along a y axis that extends vertically upward and a width of 1.05 m alo...
A: Using Newtons law and torque about the hinges
Q: Please answer question 2 and 8
A: Hello. Since you have posted multiple questions and not specified which question needs to be solved,...
Q: 2. The two attached pulleys driven by the belt are rotating as a single unit about the center shaft....
A:
Q: 1. A golf ball is hit from 4.3 m above a golfing fairway with an initial velocity of 30.0 m/s at an ...
A: Given: A golf ball is hit from 4.3 m above a golfing fairway with an initial velocity of 30.0 m/s at...
Q: An LP record rotates at 33.33 revs/min. A penny is placed flat on the record a distance 8cm from the...
A: Given, Lprecord rotates at 33.33 rev/min penny distance from center = 8 cm
Q: Chapter 12, Problem 045 In the figure, a lead brick rests horizontally on cylinders A and B. The are...
A: Fractional change in lengths of cylinder A and B are the same since the brick rests horizontally on ...
Q: A 66 kgkg woman steps onto an up-going escalator, which has an incline of 32∘∘ with respect to the h...
A:
Q: Which pair of waves could overlap to produce a wave with a higher amplitude through interference? O ...
A:
Q: 11. Coherent light of wavelength 528 nm (1 nm = 10-9 m) is sent through two parallel slits in an opa...
A:
Q: A student is trying to determine the muzzle velocity (initial) of a bullet that is too fast to measu...
A: According to the law of conservation of momentum, Here, m1, m2 denotes the mass of the bullet and b...
Q: Light of wavelength 444 nm is incident on a diffraction grating that has 8560 lines per cm. What is ...
A: mλ=dsinθmλ=1Nsinθθ=sin-1mλN
Q: An airplane wing is designed so that the speed of the air across the top of the wing is 264 m/s when...
A:
Q: damped harmanic oscillator, has damping aonstant a B = 2 Wo, that is acted upon bya driving force F ...
A: Given: Driven force F = Fo sinωt Damping Constant β=2ω Fid the equation of motion and its correspond...
Q: ultrasound physics Which of the following is a true stamen concerning spatial pulse length? Spatial...
A: The spatial pulse length is the length of pulse from front to back or length of one complete pulse t...
Q: 19.) A tetherboll is connecteal to the top Cof a pole uith long that is 2.58 m om ongle of the pole ...
A:
Q: large plate nade 345 MPa determine the O 0.0182 mm O 9.079 mm
A: Given The Plane Strain fracture toughness = 82.4MPam The Plate is exposed to a tensile stress of 345...
Q: Solve this attachment.
A: Here generalized coordinates are r and ϕ So generalized momentum would be pr and pϕ Kinetic energ...
Q: Show all steps and provide picture if possible
A: Mass of lighter fragment, ml=1.75 MeV/c2Mass of heavier fragment, mh=3.50 MeV/c21 MeV/c2=106×1.6×10-...
Q: 21 3A8.150Q y with a velo &ty of base ball is throun horizan talo A 28.0 mis in the negotie * -direc...
A:
Q: An object of height 1 cm is placed 2.5 cm in front of a concave mirror of focal length of 3 cm. (a,5...
A:
Q: Consider a circular ring of radius r as shown in figure 3, through which a current i passes:a) Calcu...
A: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question and s...
Q: As the story goes, an Aggie, a Texas Longhom and a Baylor Bear were trying to swim from one island t...
A:
Q: Dolphins rely on echolocation to be able to survive in the ocean. In a 20 °C ocean, a dolphin produc...
A:
Q: A 30 kg block is resting on a flat horizontal table On the top of the block is a 15.0 kg block to wh...
A:
Q: A 35.0g steel ball initially moving at 10.0 m/s in the positive x-direction collides completely elas...
A:
Q: In order better to map the surface features of the Moon, a 361 kgimaging satellite is put into circu...
A: Given Mass (m) = 361 kg Radius (R) = 1740 km Moon mass (M) = 7.36×1022 kg Dis...
Q: The two particles are moving to the right. Particle 1 catches up with particle 2 and collides with ...
A: In the collisions, the Linear momentum is conserved Using conservation of linear momentum Pinitial ...
Q: Workers around jet aircrafts typically wear protective devices over their ears, as the sound level o...
A:
Q: . (II) Estimate the wavelength for an n = 3 to n = 2 transition in iron (Z = 26).
A: Wavelength for the given transition 1λ=RZ-121n12-1n22λ=1RZ-121n12-1n22-1
Q: 1. What are the main components of a nuclear reactor?
A: Answer: Introduction: It is here to introduce that the main components of a nuclear reactor are di...
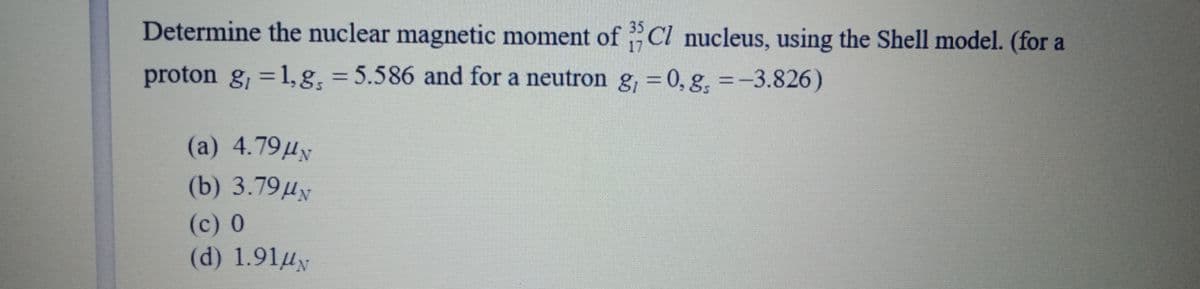
Step by step
Solved in 2 steps with 1 images
