Determine the specific gravity of the gas in TABLE 1. Note: Convert the weight fraction to mole fraction first and state your assumptions
Determine the specific gravity of the gas in TABLE 1. Note: Convert the weight fraction to mole fraction first and state your assumptions
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Determine the specific gravity of the gas in TABLE 1.
Note: Convert the weight fraction to mole fraction first and state your assumptions
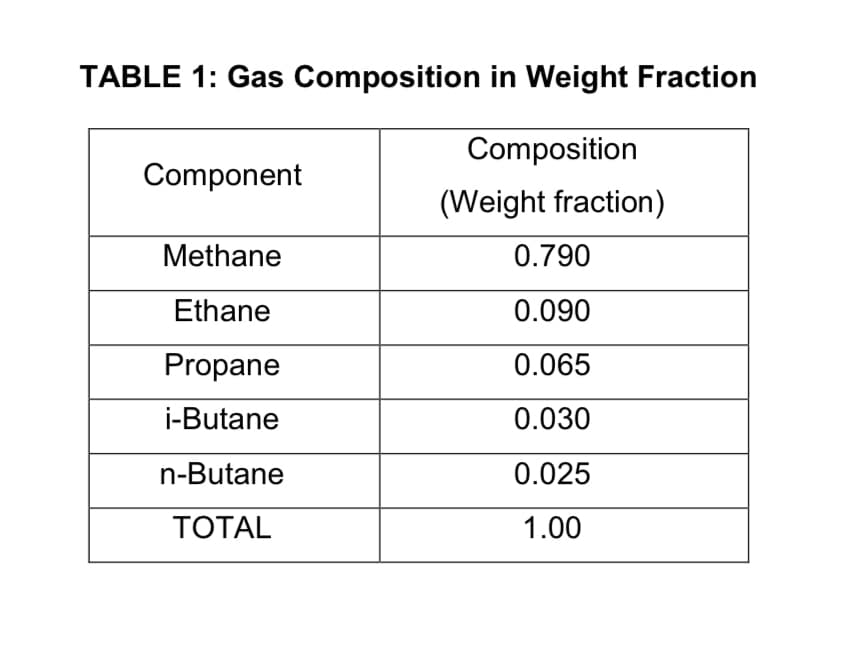
Transcribed Image Text:TABLE 1: Gas Composition in Weight Fraction
Composition
Component
(Weight fraction)
Methane
0.790
Ethane
0.090
Propane
0.065
i-Butane
0.030
n-Butane
0.025
TOTAL
1.00
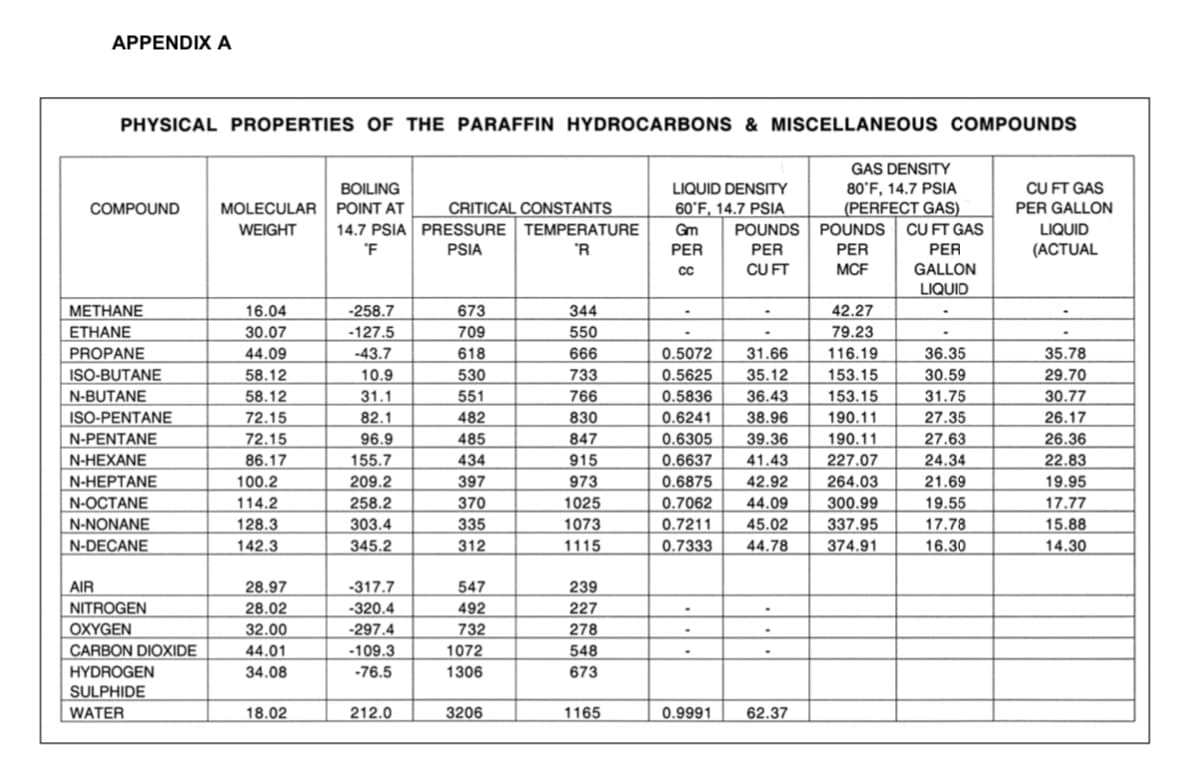
Transcribed Image Text:APPENDIX A
PHYSICAL PROPERTIES OF THE PARAFFIN HYDROCARBONS & MISCELLANEOUS COMPOUNDS
GAS DENSITY
CU FT GAS
PER GALLON
BOILING
LIQUID DENSITY
80°F, 14.7 PSIA
(PERFECT GAS)
POUNDS
MOLECULAR POINT AT
CRITICAL CONSTANTS
14.7 PSIA PRESSURE TEMPERATURE
PSIA
COMPOUND
60°F, 14.7 PSIA
WEIGHT
Gm
CU FT GAS
POUNDS
PER
CU FT
LIQUID
PER
GALLON
"F
"R
PER
PER
(ACTUAL
MCF
LIQUID
МЕТHANE
16.04
-258.7
673
344
42.27
ETHANE
30.07
-127.5
709
550
79.23
116.19
PROPANE
-43.7
618
666
0.5072
35.78
31.66
35.12
36.43
44.09
36.35
ISO-BUTANE
58.12
10.9
530
733
0.5625
153.15
153.15
190.11
30.59
31.75
27.35
27.63
29.70
30.77
26.17
N-BUTANE
58.12
31.1
551
766
0.5836
ISO-PENTANE
72.15
82.1
482
830
0.6241
38.96
N-PENTANE
72.15
96.9
485
847
0.6305
39.36
190.11
227.07
26.36
N-HEXANE
86.17
155.7
434
915
0.6637
41.43
24.34
22.83
N-HEPTANE
209.2
258.2
303.4
345.2
397
973
0.6875
42.92
264.03
21.69
19.55
17.78
16.30
100.2
19.95
N-OCTANE
114.2
370
335
1025
0.7062
0.7211
44.09
300.99
337.95
374.91
17.77
N-NONANE
N-DECANE
128.3
1073
45.02
15.88
142.3
312
1115
0.7333
44.78
14.30
AIR
28.97
-317.7
547
239
NITROGEN
28.02
-320.4
492
227
OXYGEN
32.00
-297.4
732
278
CARBON DIOXIDE
44.01
-109.3
1072
548
HYDROGEN
34.08
-76.5
1306
673
SULPHIDE
WATER
18.02
212.0
3206
1165
0.9991
62.37
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The