distillation column is to be designed to produce 99.5% pure oxygen from air
distillation column is to be designed to produce 99.5% pure oxygen from air
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Relatively pure oxygen is required in a large number of applications ranging from
incubators for premature babies in neo-natal intensive care units to the use of
oxygenation reactions in the production of various chemicals. An attractive way of
generating this high purity oxygen is to use a small distillation column, with oxygen
removed in the bottoms. The distillate, which is simply a more concentrated nitrogen
stream, can be directly discharged into the atmosphere. The column obviously operates
at very low temperatures (typically referred to as cryogenic distillation) to attain the
desired VLE for the O2-N2 system.
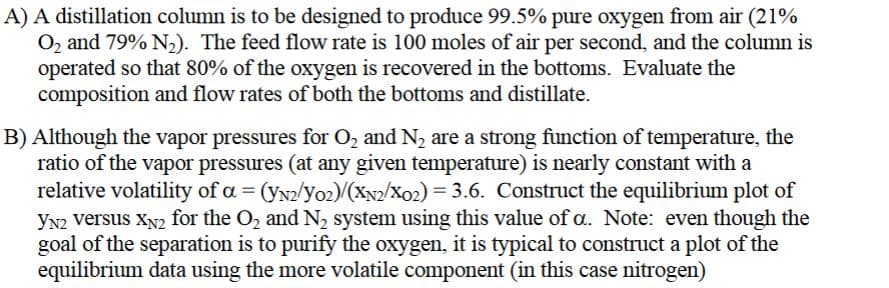
Transcribed Image Text:A) A distillation column is to be designed to produce 99.5% pure oxygen from air (21%
O₂ and 79% N₂). The feed flow rate is 100 moles of air per second, and the column is
operated so that 80% of the oxygen is recovered in the bottoms. Evaluate the
composition and flow rates of both the bottoms and distillate.
B) Although the vapor pressures for O₂ and N₂ are a strong function of temperature, the
ratio of the vapor pressures (at any given temperature) is nearly constant with a
relative volatility of a = (YN2/Y02)/(XN2/X02) = 3.6. Construct the equilibrium plot of
YN2 versus XN2 for the O₂ and N₂ system using this value of a. Note: even though the
goal of the separation is to purify the oxygen, it is typical to construct a plot of the
equilibrium data using the more volatile component (in this case nitrogen)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The