Dry reforming is a novel reaction concept combines two greenhouse gases (CO2 and CH4) to produce CO and H2 (termed synthesis gas since these two molecules can be used as the building blocks to produce many other petrochemical chemicals). The reaction follows: CO2 + CH4 S 2H2 + 2CO (50 – 100 atm, 250 °C) The thermochemical data for each of the species at 1000 K are: CO2 CH4 Н CO AH;° (kJ/mol) -360.11 -36.42 20.68 -88.85 s° (J/mol-K) 166.22 269.30 249.95 234.53 (1) What are the heat of reaction, entropy of reaction, and the Gibbs free energy of reaction at this temperature (i.e., 1000 K)? Please include the appropriate units. (2) What is the gas phase equilibrium constant at 1000 K? (3) If the reaction is carried out at 0.5 atm and equal molar of carbon dioxide and methane are fed, what is the equilibrium conversion under this condition (0.5 atm and 1000 K)? (4) To increase the equilibrium conversion at 1000 K, should we increase or decrease the reaction pressure? Why? Report the equilibrium conversion at a different pressure (other than 0.5 atm) to support your answer. (5) A researcher followed the above equilibrium analysis and attempted to carry out the reaction at the same temperature (i.e., 1000 K) and pressure (i.e., 0.5 atm) in a plug flow reactor (i.e., by feeding CO2 and CH4 into a hollow tube). However, this researcher quickly found that the actual conversion is much smaller than the calculated equilibrium conversion. Can you suggest a hypothesis why this is happening? What potential strategy you would recommend to help increase the reaction conversion?
Dry reforming is a novel reaction concept combines two greenhouse gases (CO2 and CH4) to produce CO and H2 (termed synthesis gas since these two molecules can be used as the building blocks to produce many other petrochemical chemicals). The reaction follows: CO2 + CH4 S 2H2 + 2CO (50 – 100 atm, 250 °C) The thermochemical data for each of the species at 1000 K are: CO2 CH4 Н CO AH;° (kJ/mol) -360.11 -36.42 20.68 -88.85 s° (J/mol-K) 166.22 269.30 249.95 234.53 (1) What are the heat of reaction, entropy of reaction, and the Gibbs free energy of reaction at this temperature (i.e., 1000 K)? Please include the appropriate units. (2) What is the gas phase equilibrium constant at 1000 K? (3) If the reaction is carried out at 0.5 atm and equal molar of carbon dioxide and methane are fed, what is the equilibrium conversion under this condition (0.5 atm and 1000 K)? (4) To increase the equilibrium conversion at 1000 K, should we increase or decrease the reaction pressure? Why? Report the equilibrium conversion at a different pressure (other than 0.5 atm) to support your answer. (5) A researcher followed the above equilibrium analysis and attempted to carry out the reaction at the same temperature (i.e., 1000 K) and pressure (i.e., 0.5 atm) in a plug flow reactor (i.e., by feeding CO2 and CH4 into a hollow tube). However, this researcher quickly found that the actual conversion is much smaller than the calculated equilibrium conversion. Can you suggest a hypothesis why this is happening? What potential strategy you would recommend to help increase the reaction conversion?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
i need help with all parts in this question
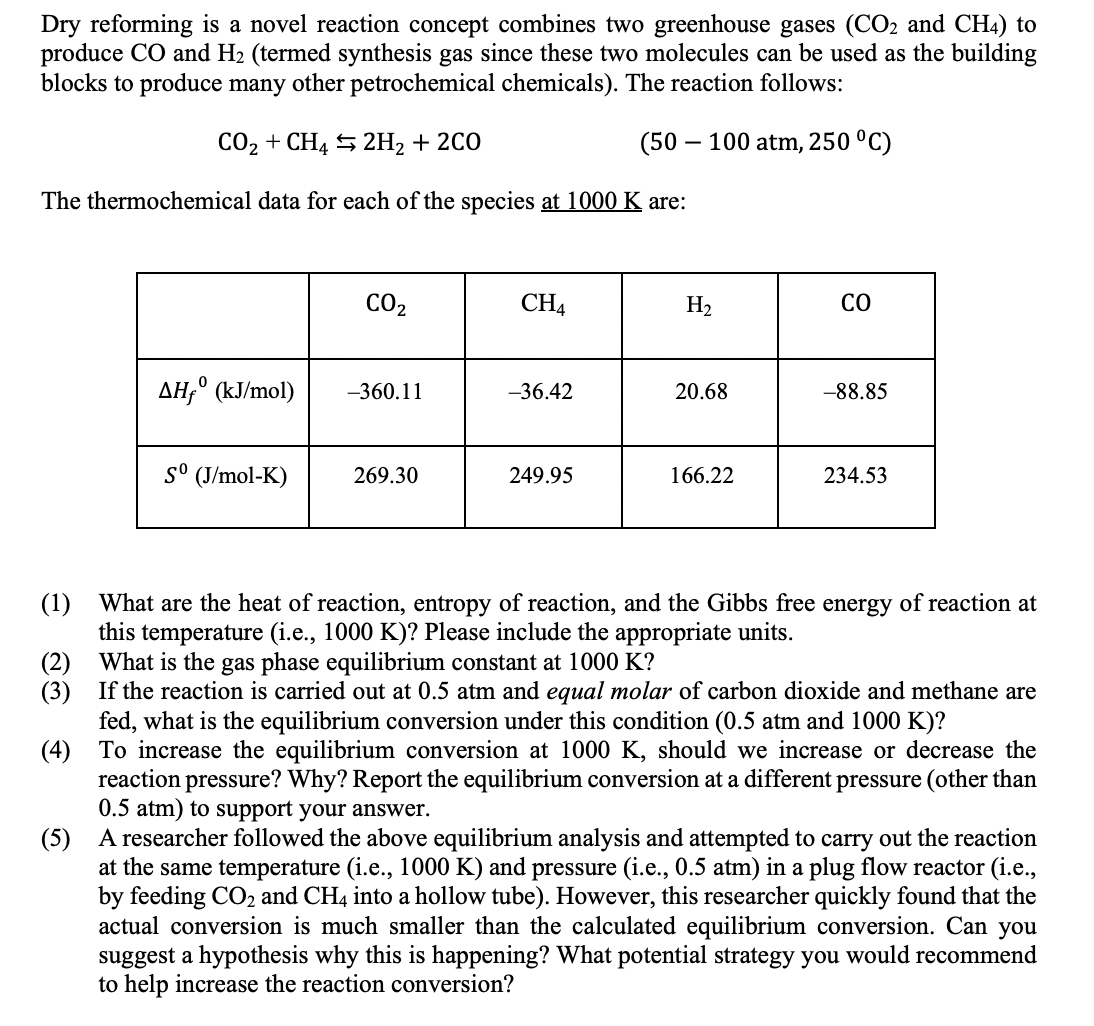
Transcribed Image Text:Dry reforming is a novel reaction concept combines two greenhouse gases (CO2 and CH4) to
produce CO and H2 (termed synthesis gas since these two molecules can be used as the building
blocks to produce many other petrochemical chemicals). The reaction follows:
CO2 + CH4 5 2H2 + 2CO
(50 – 100 atm, 250 °C)
-
The thermochemical data for each of the species at 1000 K are:
CO2
CH4
H2
CO
AH;° (kJ/mol)
-360.11
-36.42
20.68
-88.85
(J/mol-K)
269.30
249.95
166.22
234.53
(1) What are the heat of reaction, entropy of reaction, and the Gibbs free energy of reaction at
this temperature (i.e., 1000 K)? Please include the appropriate units.
(2) What is the gas phase equilibrium constant at 1000 K?
(3) If the reaction is carried out at 0.5 atm and equal molar of carbon dioxide and methane are
fed, what is the equilibrium conversion under this condition (0.5 atm and 1000 K)?
(4) To increase the equilibrium conversion at 1000 K, should we increase or decrease the
reaction pressure? Why? Report the equilibrium conversion at a different pressure (other than
0.5 atm) to support your answer.
(5) A researcher followed the above equilibrium analysis and attempted to carry out the reaction
at the same temperature (i.e., 1000 K) and pressure (i.e., 0.5 atm) in a plug flow reactor (i.e.,
by feeding CO2 and CH4 into a hollow tube). However, this researcher quickly found that the
actual conversion is much smaller than the calculated equilibrium conversion. Can you
suggest a hypothesis why this is happening? What potential strategy you would recommend
to help increase the reaction conversion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The