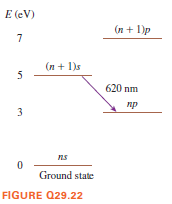
E (eV) (n + 1)p 7 (п + 1)s 5 620 nm пр 3 ns Ground state FIGURE Q29.22
Q: 500 mm Situation 1. A hand truck is used to move two kegs, each of mass 40 kg. Neglecting the mass o...
A:
Q: A car traveling in a straight line with an initial velocity of 8.8m/s accelerates at a rate of 3.0m/...
A: Given: Initial velocity, u = 8.8 m/s Final velocity, v = 23.8 m/s Acceleration, a = 3 m...
Q: Color f(1014 Hz) 1 (nm) Red 4.41 680 Orange Yellow Green Blue Violet
A: The expression to solve for the wavelength is as follows: λ=cf 1 The wavelength associa...
Q: a. Find the required applied force to move this block up the ramp at constant speed. b. Calculate th...
A: Given Mass = 2000 kg Angle =26° Friction = 0.4 Height = 100 m
Q: Calculate how many moles of He gas are needed to have statistical certainty that one atom of Helium ...
A: The probability (P) of finding one He-atom out of a total of N He-atoms (or n moles of He) may be gi...
Q: Electrons are accelerated through a potential difference of 750 kV, so that their kinetic energy is ...
A: Given Potential difference = 750 kV Kinetic energy =7.50×105 eV
Q: Sand moves without slipping at 6.0 m/sm/s down a conveyer that is tilted at 15∘∘. The sand enters a ...
A: The equation of motion for projectile motion is given by Where x = Represents horizontal motion, y ...
Q: Military rifles have a mechanism for reducing the recoil forces of the gun on the person firing it. ...
A:
Q: What is the right ascension in hours, minutes, and seconds of a star at RA 239.768°?
A:
Q: (a) What is the heat capacity Cv of a three-dimensional cubic lattice of atoms at room temperature? ...
A: (a) Introduction: Heat capacity or thermal capacity is a physical property of matter, defined as the...
Q: What is the kinetic energy of (a) an electron having a momentum of 40 GeV/c? (b) a proton having a m...
A: Given, Momentum of electron is 40 GeV/c Momentum of proton is 40 Gev/c
Q: (a) Using Gauss's law calculate the electric field above a charged conductor. The system is shown in...
A: We need to find the electric field E for the given surface having a surface charge density σ. To sol...
Q: Please answer Parts F,G and H of question 4.
A: Given: Initial velocity is vi=94.322m/s θ=43.0o Initial velocity component along x axis is vix=vi*co...
Q: Let the total number of neutrons be Nn, the number of protons be Np, and N = Nn + Np. Let the fracti...
A: Given Let the total number of neutrons be Nn, the number of protons be Np, and N = Nn + Np. Let the ...
Q: Please use the data given to answer all parts of question 1. thank you in advanced!
A: In x-direction (eastwards) : ax=vxt=8.00 ms-13.90 s=2.05ms-2 In negative y-direction (south) :ay=vyt...
Q: Superman throws a 2400 N boulder at an adversary. What horizontalforce must Superman apply to the bo...
A: The weight of the boulder is W = mg Here, m is the mass of the boulder, g is the acceleration due to...
Q: A speedboat is cruising at a speed of 12.0 m/s in still water when it shuts off its engines and coas...
A: (a) From the given expression v=vie-ct-ct=lnvvic=-1tlnvvic=-121.0 sln5.00 m/s12.0 m/s=4.17×10-2 /s
Q: A ball is hanging from a long string that is tied to the ceiling of a train car traveling eastward o...
A: The train is moving with uniform velocity with respect to the ground. The rate of change of velocity...
Q: Please do J, K, and L
A: According to equation (2) x^ = h2mωa^+ + a^ The unit of Planck's constant is Kgm2s-1, the unit of m...
Q: The Mössbauer effect was discovered using the decay of the 0.12939 MeV second excited state of 19'Ir...
A: The Mossbauer Effect is a process in which a nucleus, which is in an excited state emits or else whi...
Q: In the circuit in (Figure 1), a 20-ohm resistor sits inside 114 g of pure water that is surrounded b...
A:
Q: Differentiate between grains and grain boundary in terms of following a. Energy level b. Accumulatio...
A: A grain is a bunch of crystals arranged in the same direction when small crystals growth they can fo...
Q: A car and a truck start from rest at the same instant, with thecar initially at some distance behind...
A:
Q: When a batted baseball moves with air drag, when does the ball travel a greater horizontal distance?...
A: The baseball moves until it reaches the ground (ignoring the subsequent motion). The air drag acts a...
Q: Consider the incline shown in the figure with inclination angle θ and height h. The coefficient of k...
A:
Q: Two pressure sensors are located at the seafloor: one is 100 m and the other is 500 m off the coastl...
A: Given: The distance of two pressure sensors are 100 m and 500 m respectively. The pressure amplitude...
Q: You direct a beam of 0.154 nm x rays at certain planes of a silicon crystal. As you increase the ang...
A: Part-a Given: Wavelength of the beam λ = 0.154 nm. Angle of diffraction θ = 34.5o.
Q: the diaphragm of a loud speaker is generating a sound by moving back and forth in simple harmonic mo...
A: Given angular frequency of diaphragm 7.5x104rad/s.
Q: Consider the following nuclear reaction B → Be + 'e + v and determine the Q value or total energy re...
A:
Q: The amount of charge on the plates of a 300 x 10-6 F capacitor which is connected to a 12.0 V sourc...
A: the amount of charges on the capacitor plates, Q=CV
Q: Fluorescent lights often use an inductor, called a ballast, to limit the current through the tubes. ...
A: A resistor loses energy in the form of heat when current flows through it. Heat lost is given by H=i...
Q: A small block has constantacceleration as it slides downa frictionless incline. The block isreleased...
A:
Q: Circuit
A: Formula used - The equivalent resistance of a series combination of resistors is given by, Rs = R1 ...
Q: Two people are pushing horizontally on a crate of mass 50 kg. One person is pushing from the right w...
A: The kinetic friction force on the object is given by Where = Coefficient of kinetic friction, FN =...
Q: Why is it difficult to accurately state how long a specific comet is?
A: it has been believing that the comets are the remnants of the materials formed the solar system 4....
Q: Please answer parts A and B. The pair at the bottom of the page that is circled. Use all data given ...
A: Given values, d2=300 m t3=170 s
Q: A light string can support a stationary hanging load of 22.4 kg before breaking. An object of mass m...
A: In the circular motion of an object, centrifugal force acts on the system. The centrifugal force is ...
Q: You have available a 550 watts pump with 70% efficiency. Is it possible to use this pump to transfer...
A: Given : Power of the pump(P) = 550W with 70% efficiency ⇒ P = 550 x 70100 ...
Q: What is the spectroscopic term symbol for gallium in its ground state? Explain.
A: The electronic configuration of gallium ends with the following subshells, 3d104s24p1 This indicates...
Q: A telescope on Earth is taking measurements of a nearby star six months apart. The telescope is limi...
A: Given: A telescope on Earth is taking measurements of a nearby star six months apart. The telescope ...
Q: What fraction of 5-MeV - particles will be scattered through angles greater than 8° from a gold foil...
A: Given: The value of gold foil is Z = 79, density=19.3 g/cm3
As shown the energy levels of a hypothetical atom.
a. What minimum kinetic energy (in eV) must an electron have to collisionally excite this atom and cause the emission of a 620 nm photon? Explain.
b. Can an electron with K = 6 eV cause the emission of 620 nm light from this atom? If so, what is the final kinetic energy of the electron? If not, why not?
c. Can a 6 eV photon cause the emission of 620 nm light from this atom? Why or why not?
d. Can a 7 eV photon cause the emission of 620 nm light from this atom? Why or why not?
Trending now
This is a popular solution!
Step by step
Solved in 3 steps
