E (eV) n=3 -2.0 n= 2- -3.0 n=1 -6.5 FIGURE P29.59
Q: Light of free-space wavelength 20 = 0.75 um is guided by a thin planar film of thickness d=2.5 μm an...
A: As per guidelines, the first three sub-parts have been answered. Kindly post the remaining questions...
Q: a) Find the capacitance of the capacitor. b) Find the charge stored on the plates of the capacitor i...
A: The capacitance of the parallel plate capacitor is given by Where k = dielectric constant, A = Area...
Q: Discuss the advantages of digital radiography vs conventional film?
A: Digital radiography is a non-destructive technique used for the diagnosis of internal organs and it ...
Q: A muon is a lepton that is a higher-mass (rest mass 105 MeV/c2) sibling to the electron. Muons are p...
A: The kinetic energy of a particle moving with a speed is K=γ-1mc2 The mass of muon is m=105 MeV/c2 Th...
Q: Consider the mass spectrometer shown schematically in Figure. The electric field between the plates ...
A: Hey, since there is multiple subpart question posted, we will answer first three questions. If you w...
Q: Calculate the mean (expectation) values of the coordinate and momentum for a particle in a ld infini...
A:
Q: How can I get the solutions and answer to this problem? A piston cylinder's weight is 1.5 times the ...
A: Let W and F denote the weight of the gas and the force applied by the atmospheric pressure (Patm) on...
Q: The three forces shown in the figure act on an object. What fourth force must act on the object so t...
A: The components of the force shown in the first quadrant is as follows: Fx=4 NFy=4 N ...
Q: In a downhill ski race surprisingly little advantage is gained by getting a running start. This is b...
A: Given: The displacement of the skier is 65 m. The slope of the hill is 25°. a. The initial speed is ...
Q: Two massless strings of length L=2 m are attached to a vertical rod at points a distance L= 2.0 m ap...
A:
Q: Could you please help me on #78? thanks so much.
A: The electric field due to infinite non-conducting plane sheet be calculated as, E1=σ2ε0=2 μC.m-228.8...
Q: Consider a homogeneous billiard ball of mass m and radius R thatmoves on horizontal table. Gravity a...
A: (a) Given: The mass of the billiard ball is m. The radius of the billiard ball is R. Introduction: P...
Q: A 60.0-kg person rides in an elevator while standing on a scale. The scale reads 400. N. What is the...
A: Mass of person m=60 kg The apparent weight of the person (scale reading) R=400 N
Q: Q2: Express the vector field W = (x² – y²)ay + xza, in the (a) Cylinderical coordinate at P(p = 6, 9...
A: Given , The vector field, W→=x2-y2ay^+xzaz^ a. In cylindrical co ordinate x=ρcosφy=ρsinφz=z Thus W→...
Q: A thermometer is removed from a room where thetemperature is 70° F and is taken outside, where the a...
A: The differential equation for Newton’s cooling law can be expressed as, Here Ts is the temperature ...
Q: 14. A car travels at a constant velocity of 30 m/s. How far does it travel in 60 seconds?
A: Velocity = 30 m/s Time = 60 seconds
Q: 5. A ball rolls downhill with a constant acceleration of 4 m/s2. If it started from rest, how long w...
A: v=u+att=v-ua
Q: If a sunspot has a temperature of 4,270 K and the average solar photosphere has a temperature of 5,7...
A: According to Stephen Boltzmann law E = σT4 (J/s/m2) where σ is a proportionality constant equal to 5...
Q: Two plane semi-infinite slabs of the same uniform, isotropic, nonpermeable, lossless dielectric with...
A: Let us consider electric field vectors : Incident side: Ei→eik→.x→, Er→e-ik→.x→ In air gap: E+→eik...
Q: A 375 gram basketball is bounced off of a wooden board, as shown below. It is moving straight down a...
A: Given values, Mass of basketball, m=375 g=0.375 Kg time of contact, t=68.5 ms=0.0685 s Before collis...
Q: is slowly increased from zero until the block starts to slide. At what angle of inclination 0 = 0, d...
A: The various forces acting on the block are shown in the figure mgsinθ=f -(i)mgcos θ=N -(ii)...
Q: What is Pong's initial position? cm What is Pong's initial velocity? cm/s What is Pong's acceleratio...
A: Pong's Initial position can be determined by , putting t=0 in the above equation .which gives , ...
Q: Find the average energy E for: (a) An n-state system, in which a given state can have energy 0, e, 2...
A: Solution: To calculate the Average energy we have to write the partition function as below: z=∑i...
Q: My answer was wrong
A: Use kinematic expression to find the time elapsed v=u+att=v-ua=27.4 m/s-9.4 m/s3.8 m/s2=4.74 s
Q: Write a note on “ENTROPY”.
A: Entropy can be defined as the measure of randomness or molecular disorderness of a system. It is an ...
Q: Advanced Physics Question
A: Given coordinates of the of the point charge: r = (x, y) = (0.5 , 0.3) The distance of the point cha...
Q: Ch.5 Problem 86
A: The expression for the force acting on the spring is as follows: F1=kx1k=F1x1k=m1gx1 ...
Q: A cube has sides of length L = 20cm. It is placed with its one corner at the origin. The electric fi...
A: Given information: The length of the side of the cube (l) = 20 cm The electric field = 6i-3j N/c The...
Q: 2. A block with mass m = 14.2 kg slides down an inclined plane of slope angle 37.7o with a constant ...
A: Given : The mass of the block m = 14.2 kg The angle of the inclined plane with respect to the surfac...
Q: You leave on a mission to a distant star. Your craft accelerates at 0.95g for 30 days. From your poi...
A: Let the craft’s acceleration for the given time (t0) be denoted by a. Evaluate its speed (v) at the ...
Q: An observer is stationary in the inertial system S. A car is moving in the x-direction with speed v ...
A: The velocity of the object in frame S' and S are related as ...
Q: The most probable distribution of particles is the one with the lowest number of possible m...
A: The most probable distribution of particles is the one in which the entropy of the system is maximum...
Q: Consider the figure below. 45.0° 45.0° 60.0 N 60.0 N (a) What is the resultant force exerted by the ...
A: T1 = 60.0 N T2 = 60.0 N W = Weight of the light
Q: Draw energy-level diagrams, similar to as shown, for all A = 10 nuclei listed in Appendix C. Show al...
A: Given: mass number is A=10 The elements with nuclei number 10 are carbon(C), Boron(B) and nitrogen(N...
Q: A child is playing on a hill with a cardboard box. Thecombined mass of child and box is ? = 120 kg. ...
A: (a) A child is playing on a hill with a cardboard box. Thecombined mass of child and box is ? = 120 ...
Q: A student records the following information about a planet. Characteristics of a Planet • Its larges...
A: Background- Saturn is the sixth planet from the Sun and the second-largest in the Solar System, afte...
Q: A uniform volume charge density of 80 uC/m is present throughout the region 8 mm 10 mm, find D, at ...
A:
Q: A block of mass M is at the top of a ramp of length L and angle 0. The block slides without friction...
A: According to the conservation law of energy, the relation between the kinetic energy and the potenti...
Q: Consider an infinitely long cylinder with radius R and axis along the z direction. The magnetization...
A: a. We know, for a magnetization M→, the volume bound current density is given by: Jb=∇×M→ the surfac...
Q: An astronaut moving with a speed of 0.75c relative to Earth measures her heart rate to be 71 beats p...
A: The frequency of the heart beat related to the time period is f=1Δt According to time dilation the t...
Q: thin lenses: a convex and a concave lenses. One goal of this system is to obtain an image that is la...
A: Focal length of the first lens is 5 cm. convex Focal length of the second lens is -5 cm. concave Th...
Q: Demonstrate that the Hamiltonian operator for a particle experiencing a harmonic potential V (x) = 1...
A: If an operator P must be Hermitian, it must satisfy the condition which is given by ∫ψ Pψ*=∫(ψ Pψ*)*...
Q: What amount of energy does it take to assemble a charge configuration consisting of 3 points charges...
A: Given: The value of the charge is 6.75×10-6 C. The three charges are separated by a distance 0.163 m...
Q: You’re windsurfing at 6.28 m/s when a gust hits, accelerating your sailboardat 0.714 m/s2at 48.8◦to ...
A: Displacement of an object moving with constant acceleration at time t is given by ..................
The first three energy levels of the fictitious element X are as shown.
a. What wavelengths are observed in the absorption spectrum of element X? Give your answers in nm.
b. State whether each of your wavelengths in part a corresponds to ultraviolet, visible, or infrared light.
c. An electron with a speed of 1.4 × 106 m/s collides with an atom of element X. Shortly afterward, the atom emits a 1240 nm photon. What was the electron’s speed after the collision? Assume that, because the atom is so much more massive than the electron, the recoil of the atom is negligible.
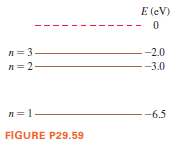
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 2 images
