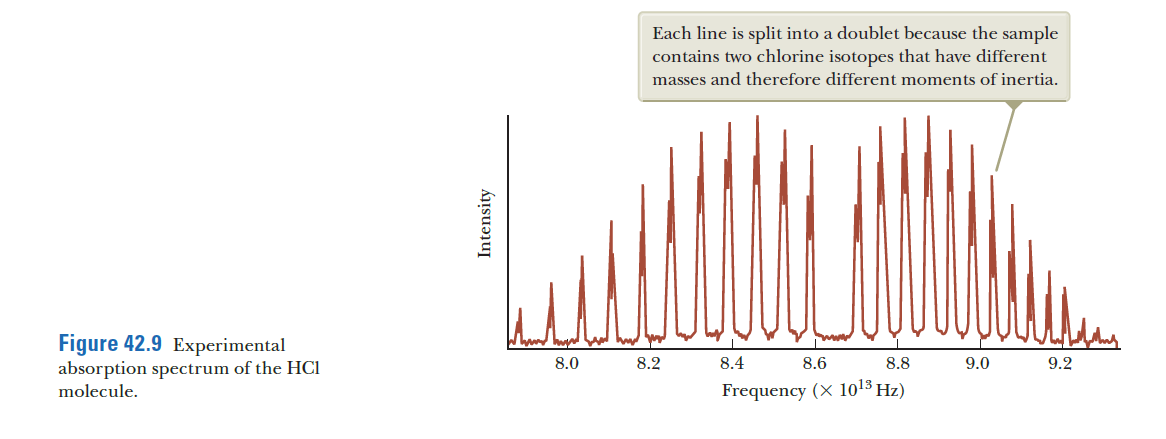
Each line is split into a doublet because the sample contains two chlorine isotopes that have different masses and therefore different moments of inertia. Figure 42.9 Experimental absorption spectrum of the HCI molecule. 8.0 8.2 8.4 8.6 8.8 9.0 9.2 Frequency (X 1013 Hz)
You are preparing to compete in the Physics Olympics. Your instructor is coaching you by providing you with challenging problems of the type you might see on an Olympics exam. He comes up with the following problem and gives you 15 minutes to solve it: Imagine a perfectly rigid HCl molecule that does not stretch as it rotates. The equilibrium separation of its ions is 0.127 5 nm. There are two isotopes for chlorine on the sample, Cl-35 and Cl-37. This results in double peaks in the molecular spectrum as shown. (a) Find an expression for the difference in the frequency between the peaks to the right of the gap as a function of the masses of the two chlorine isotopes and the quantum number J. (b) Estimate the difference in frequency numerically for J = 0, without consulting tables. Quick! Get to work!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 6 images
