Electrons with energy 54 eV were scattered by atoms on the surface of a crystal of nickel.³ The spacing between parallel rows of atoms on the surface was D= 0.215 nm. Explain why Davisson and Germer detected strong scattering at an angle ø equal to 50 degrees. %3D
Electrons with energy 54 eV were scattered by atoms on the surface of a crystal of nickel.³ The spacing between parallel rows of atoms on the surface was D= 0.215 nm. Explain why Davisson and Germer detected strong scattering at an angle ø equal to 50 degrees. %3D
Related questions
Question
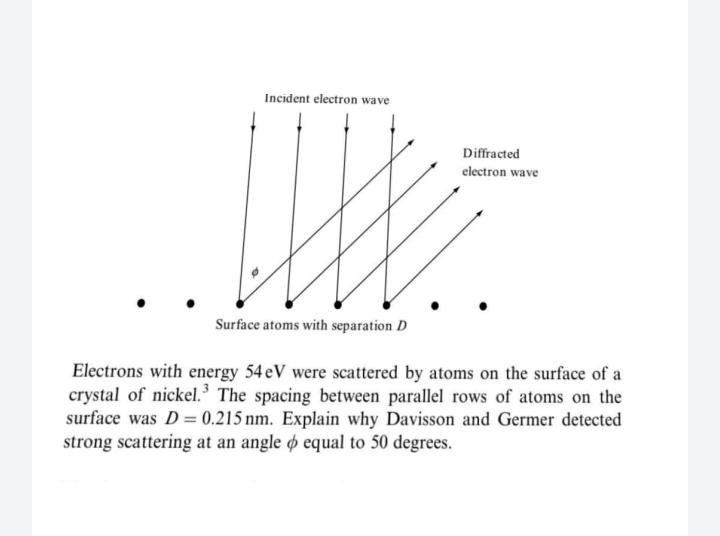
Transcribed Image Text:Electrons with energy 54 eV were scattered by atoms on the surface of a
crystal of nickel.³ The spacing between parallel rows of atoms on the
surface was D= 0.215 nm. Explain why Davisson and Germer detected
strong scattering at an angle ø equal to 50 degrees.
%3D

Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images
