Envision a solar water heater, a device which receives energy from the sun (solar radiation or thermal energy) and is used to heat water. The system is installed on the roof of a building where is receives energy from the sun at a rate of 500 Watts for every 1 m² of surface area. About 35% of the incoming thermal energy is lost to the surrounding environment. The remaining energy is absorbed by the liquid water and the temperature changes from 30 °C to 60 °C. Assuming the system is operating at steady state, find the amount of water (m) that this device can supply at 60 °C if the total surface area of the device is 3 m². Ignore any changes in kinetic energy, potential energy, or change in pressure that might occur in the system.
Envision a solar water heater, a device which receives energy from the sun (solar radiation or thermal energy) and is used to heat water. The system is installed on the roof of a building where is receives energy from the sun at a rate of 500 Watts for every 1 m² of surface area. About 35% of the incoming thermal energy is lost to the surrounding environment. The remaining energy is absorbed by the liquid water and the temperature changes from 30 °C to 60 °C. Assuming the system is operating at steady state, find the amount of water (m) that this device can supply at 60 °C if the total surface area of the device is 3 m². Ignore any changes in kinetic energy, potential energy, or change in pressure that might occur in the system.
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter11: Heat Transfer By Radiation
Section: Chapter Questions
Problem 11.56P
Related questions
Question
100%
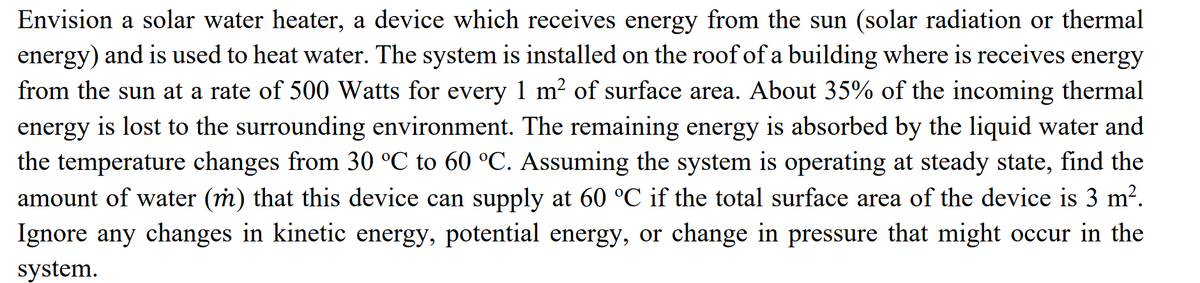
Transcribed Image Text:Envision a solar water heater, a device which receives energy from the sun (solar radiation or thermal
energy) and is used to heat water. The system is installed on the roof of a building where is receives energy
from the sun at a rate of 500 Watts for every 1 m² of surface area. About 35% of the incoming thermal
energy is lost to the surrounding environment. The remaining energy is absorbed by the liquid water and
the temperature changes from 30 °C to 60 °C. Assuming the system is operating at steady state, find the
amount of water (m) that this device can supply at 60 °C if the total surface area of the device is 3 m².
Ignore any changes in kinetic energy, potential energy, or change in pressure that might occur in the
system.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning