Estimate the diffusivity of acetic acid diffusing through air at 298 K at 2 atm using the Fuller- Schetller-Giddings method.
Estimate the diffusivity of acetic acid diffusing through air at 298 K at 2 atm using the Fuller- Schetller-Giddings method.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Problem 3 only (not a graded question)
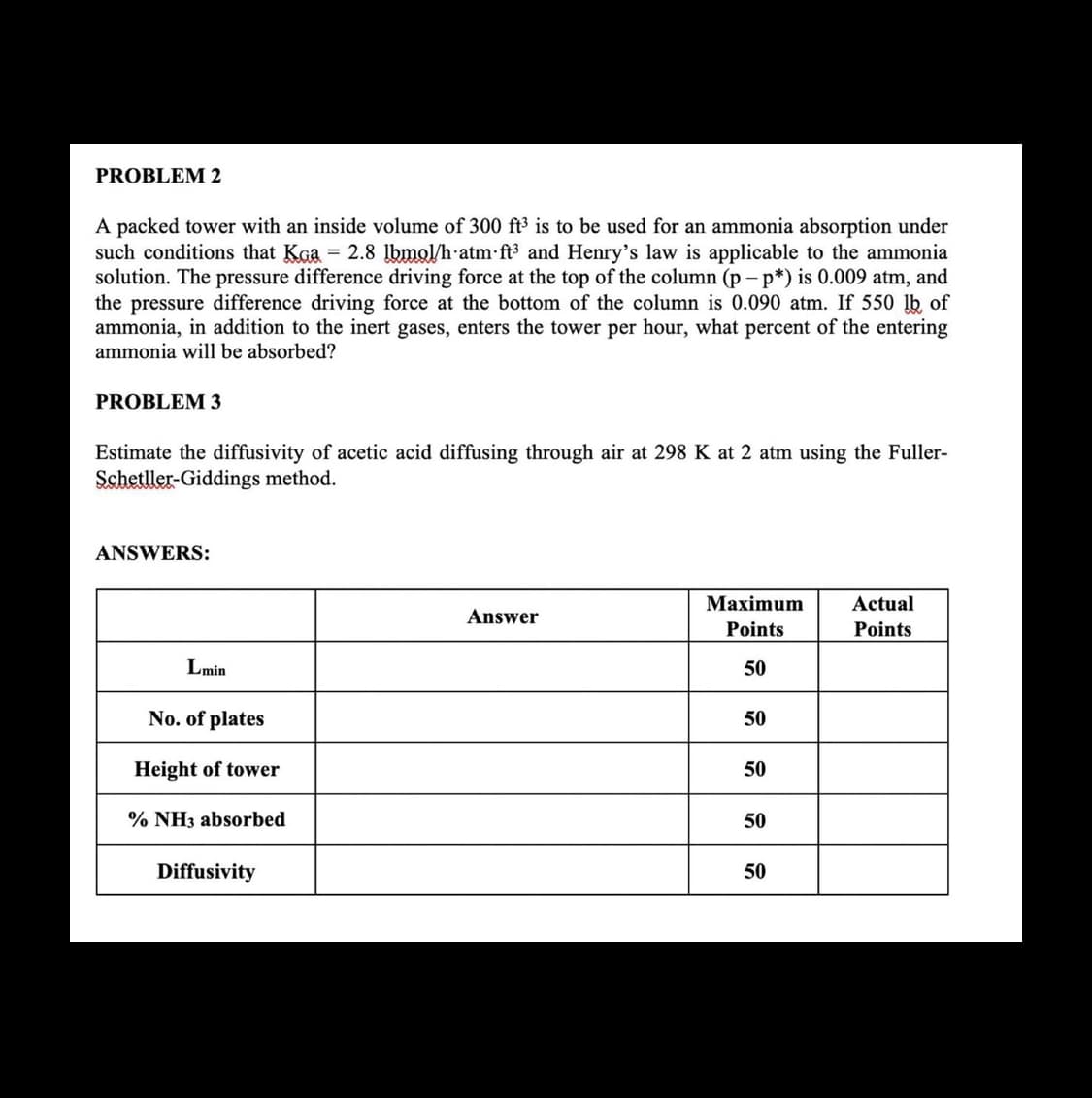
Transcribed Image Text:PROBLEM 2
A packed tower with an inside volume of 300 ft³ is to be used for an ammonia absorption under
such conditions that Kaa = 2.8 lbmol/h atm ft³ and Henry's law is applicable to the ammonia
solution. The pressure difference driving force at the top of the column (p-p*) is 0.009 atm, and
the pressure difference driving force at the bottom of the column is 0.090 atm. If 550 lb of
ammonia, in addition to the inert gases, enters the tower per hour, what percent of the entering
ammonia will be absorbed?
PROBLEM 3
Estimate the diffusivity of acetic acid diffusing through air at 298 K at 2 atm using the Fuller-
Schetller-Giddings method.
ANSWERS:
Lmin
No. of plates
Height of tower
% NH3 absorbed
Diffusivity
Answer
Maximum
Points
50
50
50
50
50
Actual
Points

Transcribed Image Text:(PROBLEM 3 ONLY)
Show your complete solution to the questions
given below. State all assumptions used
during the Draw relevant figures, if
necessary.
Do not round off during your calculations.
Unless otherwise specified, express your final
answer/s to the nearest four decimal If it is a
very small/very large number, express your
final answer/s in scientific notation to the
nearest two decimal places.
Box your final answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The