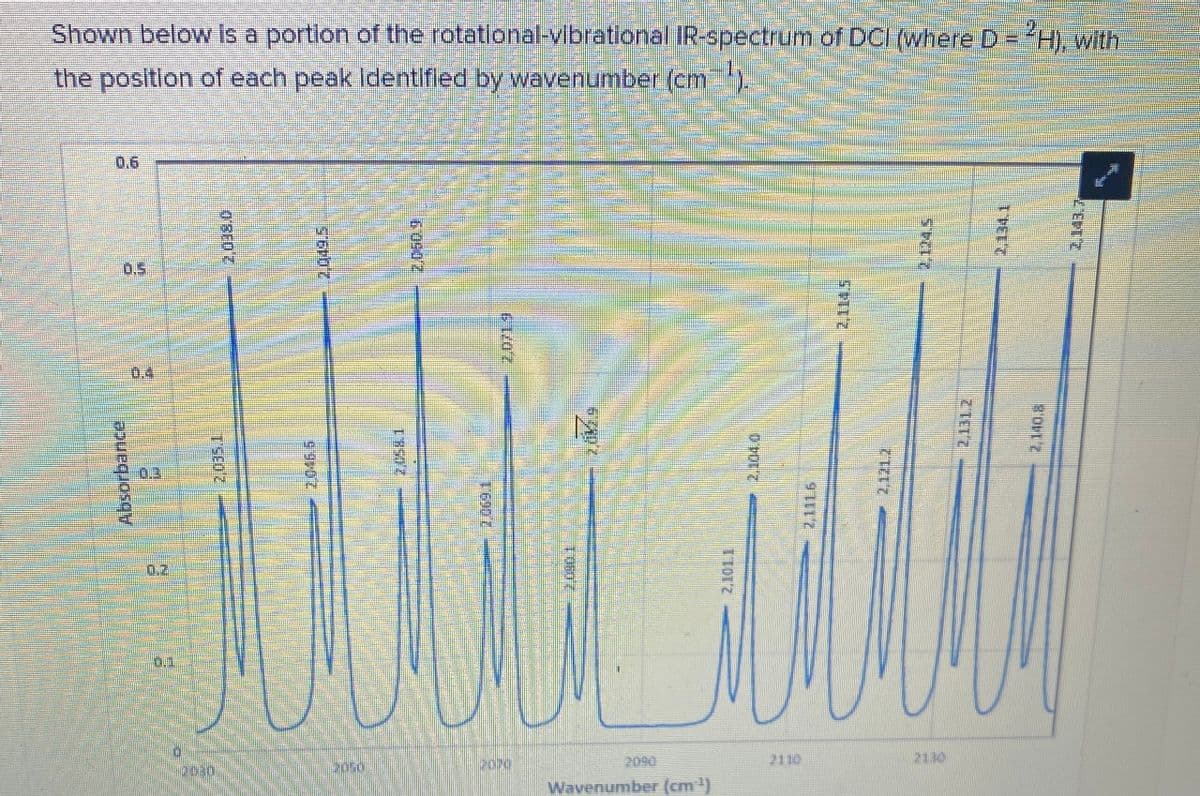
Estimate the energy difference (cm ) between the v 0, J= 0 level and the v = 1, J = 0 level. Based on this energy, calculate the force constant for the molecule.
Q: Answer question ii)
A:
Q: (b) A particle moves with position y = 2 x , where x and y are in meters. The velocity in x directio...
A: If any particle moving along X direction then the vector notion used for this will be ^i Where ^i ...
Q: kids decide to slide boxes down a long slide. the slide is inclined at 20 degrees and has a coeffici...
A:
Q: 6- Solar cell produces electric current when a. When holes move to the n side b. When photon...
A:
Q: IR Rs Vi=16V Rz VL =12V a 14 the load current Iz varies between 0-2 00 m A , find the values of Rs a...
A: Given: The input voltage is given as Vi = 16 V The voltage across the load resistor VL = 12 V The cu...
Q: The Lagrangian L = (x² + ÿ²) – mgy is defined a 2d projectile motion a) Please write Hamiltonian Can...
A: Since you have not mentioned the relevant subparts, we will be solving only the first 3 subparts.
Q: If the magnetic field faces into the wall while its magnitude is increasing, what would be the movem...
A: Given, The varying magnetic field
Q: Please type the final answer instead of writing thanks
A: (a) Number : 2.738, Units: Joule
Q: 9b
A:
Q: The ratio of the refractive indices of two adjacent layers is given by "n2/n1 = 0.77". If the wavele...
A: The relationship between refractive index of two adjacent layers and wavelength of light is given by...
Q: 1. (a) A subway train moves a total run of 700m from station1 to station 2. It accelerates and decel...
A:
Q: (advanced physics-Equations of Motion) Obtain the dynamic equations of the RR(rotarary+rotarary)...
A: The kinetic energy of the system is the sum of rotational kinetic energy and kinetic energy due to t...
Q: What is the wavelength of the photon emitted when an electron in the third excited state of neon (at...
A: Given: Neon atomic number Z=10 ni=3 and nf=1 To find: Wavelength of photon emitted when e...
Q: explain each one
A: Since we only answer up to 3 sub-parts, we’ll answer first 3. Please resubmit the question and speci...
Q: question 1
A: Given, i. Resultant is given by,
Q: 5. Calculate the efficiency of the engine that uses the cycle shown with ideal gas as a working subs...
A: Since we only answer up to 1 question, we’ll answer the 1st question only. Please resubmit the ques...
Q: what is the difference between an “energy state” and an “energy level”? why is it important to. make...
A: 1) Energy level:- The energy level is stand for a particular orbit number (n) or principal quantum n...
Q: The output voltage of a solar cell is 0.5V. Choose the number of solar cells needed to be connected ...
A: Given Total series voltage = 12 V Output voltage of a single solar cell =...
Q: 1) An infinitely long, thin rod carries a charge of coulombs per meter. Consider a square surface of...
A: The electric flux is a measure of electric field lines passing through an area. According to Gauss' ...
Q: Water at 60° F flows from the basement to the first floor through the 0.0625 – ft. diameter copper p...
A: As, you have not mentioned the subpart to be mentioned, we will solve the first subparts.
Q: 1. A child hangs onto the edge of a merry-go-round that is spinning at 30 rpm. a) In your own words...
A: (a) Motion is relative. So when comparing to frames of reference, if with respect one reference fra...
Q: For a given object position, the lateral magnification of a convex mirror is M = +0.5. If the magnit...
A: We would use the mirror formula to solve the above problem, also magnification is given by m = - v/u...
Q: 10 - Which of the following causes acid rain? a. Uranium b. Petroleum c. Natural Gas d....
A: Acid rain, also known as acid deposition, is a general phrase that refers to any type of precipitati...
Q: A thin rod of length .and uniform charge per unit length A lies along the xaxis as shown in Figure P...
A:
Q: The current segments RQ of length a and QP of length b carry current l2 and located near an infinite...
A: Given:
Q: 2) What is a -Newtonian Fluid? How can a shear stress in a fluid be defined for Newtonian Fluids. Ex...
A: Newtonian fluids are those fluid which satisfy Newton's law of viscosity. Namely as described below....
Q: A perfectly stirred-and -insulated tank, initially contains 360 L oil (p=1050 kg/m3, CP=2100 j/kg.oC...
A:
Q: . A fabric used in air-inflated structures is subjected to a biaxial loading that results in normal ...
A: To determine the change in length of side BC.
Q: Particle A of mass mA=m/2 moving along the x-axis with velocity v0 collides elastically with another...
A: Particle A of mass mA=m/2 moving along the x-axis with velocity v0 collides elastically with another...
Q: Solar input power to the PV Module is 1200W, this PV Module is producing output power of 228W then E...
A: Given: Input power of solar cell Pin=1200 W Output power of solar cell Pout=228 W To find: ...
Q: The specific heat of water = 4200 J kg–1K–1 and the latent heat of ice = 3.4 × 105 J kg-1.100 grams ...
A: The specific heat of water = 4200 J kg–1K–1 and the latent heat of ice = 3.4 × 105 J kg-1.100 grams ...
Q: What is a node in a standing wave?
A: When two waves of equal frequency and amplitude superimpose when travel in opposite direction then e...
Q: Please type the final answer instead of writting
A: Given, The radius of each sphere is R=3.6cm Center to center separation=2.7m The midpoint between th...
Q: completely lost, any help?
A: We’ll answer the first question since the exact one wasn’t specified. Please submit a new question s...
Q: How does the concept of laws of motion help the world in the future?
A: Newton's laws of motion govern the motion of bodies and how they interact with each other. Almost an...
Q: The electric field of a plane EM wave travelling along the z direction is E (E + Ey) cos(wt- kz + )....
A: The relationship between electric field and magnetic field is E/B = c Where E is electric field B is...
Q: . A fabric used in air-inflated structures is subjected to a biaxial loading that results in normal ...
A: To determine the change in length of side BC. Values can be assume though it is not given.
Q: T'he density of the Earth at any distance r from its centre approximately p = [14.2 – 11.6("/R)] x 1...
A: We’ll answer the second question since the exact one wasn’t specified. Please submit a new question ...
Q: Question 1) Briefly explain the temperature dependence of conductivity of a p-type semiconductor.
A: An extrinsic semiconductor in which impurity is added to the pure semiconductor, this impurity have ...
Q: Solve below
A: Given: The sensitivity of crystal is, The cross-sectional area of the crystal is, s = 10 cm2 = 0.00...
Q: A thin, circular coil with 41 turns that has a radius of 14.3 cm carries a current of 2.95 A. What i...
A: Concept used: Biot- Savart's law for calculating magnetic field due to current carrying element is u...
Q: 7. (a) A block whose mass m is 680 g is fastened to a spring whose spring k is 65 N/m. The block is ...
A: According to Hooks's law of elasticity, spring force is proportional to displacement of spring from ...
Q: 4) A long solenoid has n=800 turns per meter and a current I = 2 sin(wt). Inside the solenoid with a...
A: The question contains 4 sub-parts. As per our policy, we will answer the first 3. Kindly resubmit th...
Q: An ambu (artificial manual breathing unit) device is connected to a patient. Ambu and lungs of the p...
A: The kinetic theory of gases is represents the classical behaviour of thermodynamic gases. It consist...
Q: 3- In absorption cooling system, Water can never be used as working fluid. Select one: True Fals...
A:
Q: 11- In Solar module minimum value of internal resistance could be 0.05 Ohm. Select one: True Fal...
A: To explain the correct answer is given below.
Q: e Select one: O True O False
A: Given: Equation To find: Above equation is true or false
Q: The image formed by a concave mirror is inverted and located at a distance lal= 6 cm in front of it....
A: Concept used: Concave mirror is a spherical converging mirror. It can form small or large images , ...
Q: Particle is described by the wave function p = 0, x <0 and = Ae-*/L , x 2 0 a. Calculate A. b. Take ...
A:
Q: At 1773K the electrical conductivity of sapphire (single crystal of Al;Os) was found to be 1.25 mSm'...
A: At 1773K the electrical conductivity of sapphire (single crystal of Al203) was found to be 1.25 mSm"...

Trending now
This is a popular solution!
Step by step
Solved in 2 steps
