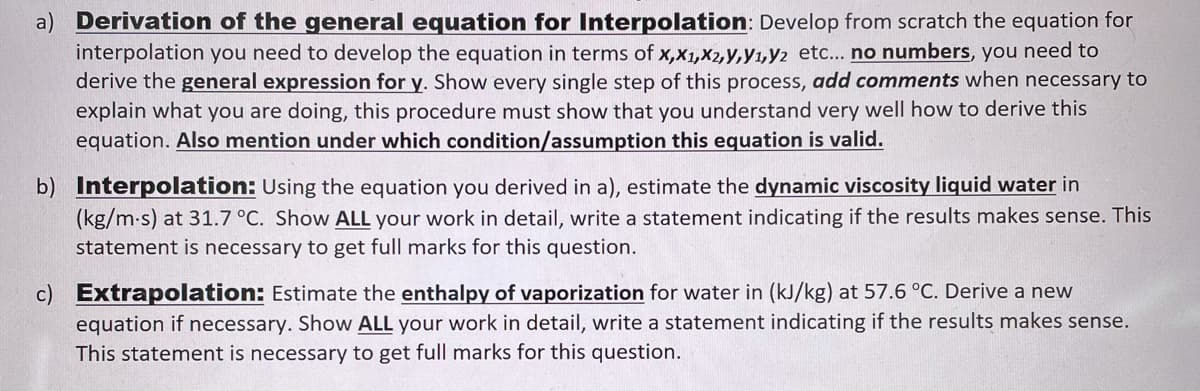
a) Derivation of the general equation for Interpolation: Develop from scratch the equation for interpolation you need to develop the equation in terms of x,x1,X2,y,Y1,Y2 etc... no numbers, you need to derive the general expression for y. Show every single step of this process, add comments when necessary to explain what you are doing, this procedure must show that you understand very well how to derive this equation. Also mention under which condition/assumption this equation is valid. b) Interpolation: Using the equation you derived in a), estimate the dynamic viscosity liquid water in (kg/m-s) at 31.7 °C. Show ALL your work in detail, write a statement indicating if the results makes sense. This statement is necessary to get full marks for this question. c) Extrapolation: Estimate the enthalpy of vaporization for water in (kJ/kg) at 57.6 °C. Derive a new equation if necessary. Show ALL your work in detail, write a statement indicating if the results makes sense. This statement is necessary to get full marks for this question.
a) Derivation of the general equation for Interpolation: Develop from scratch the equation for interpolation you need to develop the equation in terms of x,x1,X2,y,Y1,Y2 etc... no numbers, you need to derive the general expression for y. Show every single step of this process, add comments when necessary to explain what you are doing, this procedure must show that you understand very well how to derive this equation. Also mention under which condition/assumption this equation is valid. b) Interpolation: Using the equation you derived in a), estimate the dynamic viscosity liquid water in (kg/m-s) at 31.7 °C. Show ALL your work in detail, write a statement indicating if the results makes sense. This statement is necessary to get full marks for this question. c) Extrapolation: Estimate the enthalpy of vaporization for water in (kJ/kg) at 57.6 °C. Derive a new equation if necessary. Show ALL your work in detail, write a statement indicating if the results makes sense. This statement is necessary to get full marks for this question.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Transcribed Image Text:a) Derivation of the general equation for Interpolation: Develop from scratch the equation for
interpolation you need to develop the equation in terms of x,x1,X2,y,y1,Y2 etc... no numbers, you need to
derive the general expression for y. Show every single step of this process, add comments when necessary to
explain what you are doing, this procedure must show that you understand very well how to derive this
equation. Also mention under which condition/assumption this equation is valid.
b) Interpolation: Using the equation you derived in a), estimate the dynamic viscosity liquid water in
(kg/m-s) at 31.7 °C. Show ALL your work in detail, write a statement indicating if the results makes sense. This
statement is necessary to get full marks for this question.
c) Extrapolation: Estimate the enthalpy of vaporization for water in (kJ/kg) at 57.6 °C. Derive a new
equation if necessary. Show ALL your work in detail, write a statement indicating if the results makes sense.
This statement is necessary to get full marks for this question.
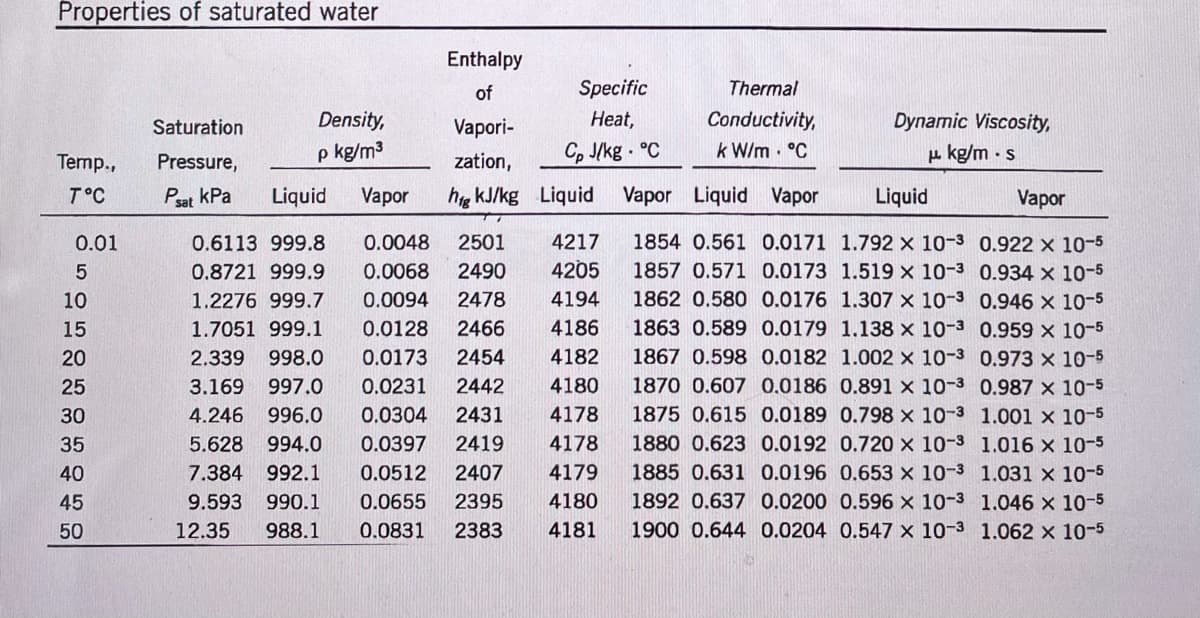
Transcribed Image Text:Properties of saturated water
Enthalpy
of
Specific
Thermal
Density,
Нeat,
Conductivity,
k W/m . °C
Saturation
Vapori-
Dynamic Viscosity,
p kg/m3
Cp J/kg . °C
u. kg/m · s
Temp.,
Pressure,
zation,
T°C
Psat kPa
Liquid
Vapor
hg kJ/kg Liquid
Vapor Liquid Vapor
Liquid
Vapor
0.01
0.6113 999.8
0.0048
2501
4217
1854 0.561 0.0171 1.792 x 10-3 0.922 x 10-5
4205
1857 0.571 0.0173 1.519 x 10-3 0.934 x 10-5
1862 0.580 0.0176 1.307 x 10-3 0.946 x 10-5
0.8721 999.9
0.0068
2490
10
1.2276 999.7
0.0094
2478
4194
15
1.7051 999.1
0.0128
2466
4186
1863 0.589 0.0179 1.138 x 10-3 0.959 x 10-5
20
2.339
998.0
0.0173
2454
4182
1867 0.598 0.0182 1.002 x 10-3 0.973 x 10-5
25
3.169
997.0
0.0231
2442
4180
1870 0.607 0.0186 0.891 x 10-3 0.987 x 10-5
30
4.246
996.0
0.0304
2431
4178
1875 0.615 0.0189 0.798 x 10-3 1.001 x 10-5
35
5.628
994.0
0.0397
2419
4178
1880 0.623 0.0192 0.720 x 10-3 1.016 x 10-5
7.384
1885 0.631 0.0196 0.653 x 10-3 1.031 × 10-5
1892 0.637 0.0200 0.596 x 10-3 1.046 x 10-5
1900 0.644 0.0204 0.547 x 10-3 1.062 x 10-5
40
992.1
0.0512
2407
4179
45
9.593
990.1
0.0655
2395
4180
50
12.35
988.1
0.0831
2383
4181
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The