Example 9.5-3: The dehydrogenation of ethanol to form acetaldehyde is carried out in a continuous adiabatic reactor. Ethanol vapor is fed to the reactor at 400 °C, and a conversion of 30% is obtained. Calculate the product Temperature. CPs.sto 48 có xlo 7 +2.38 x 10" T Solution FO 05 x 10 872 C₂H5OH(v) -> > CH3CHO(v) + H₂(g) 100 mol C₂H5OH(v) 400°C C2H5OH, CHỈ CHO C REACTOR H₂ (a 70 mol C₂H5OH(v) 30 mol CH3CHO(v) 30 mol H₂(g) Tad (°C)
Example 9.5-3: The dehydrogenation of ethanol to form acetaldehyde is carried out in a continuous adiabatic reactor. Ethanol vapor is fed to the reactor at 400 °C, and a conversion of 30% is obtained. Calculate the product Temperature. CPs.sto 48 có xlo 7 +2.38 x 10" T Solution FO 05 x 10 872 C₂H5OH(v) -> > CH3CHO(v) + H₂(g) 100 mol C₂H5OH(v) 400°C C2H5OH, CHỈ CHO C REACTOR H₂ (a 70 mol C₂H5OH(v) 30 mol CH3CHO(v) 30 mol H₂(g) Tad (°C)
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Transcribed Image Text:Ref
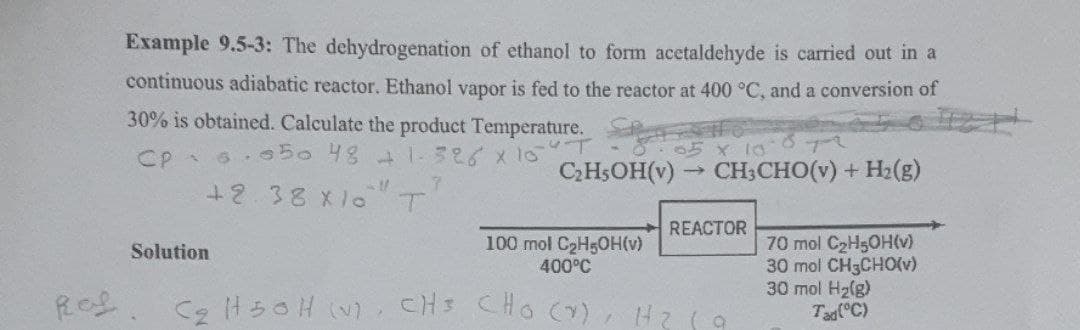
Example 9.5-3: The dehydrogenation of ethanol to form acetaldehyde is carried out in a
continuous adiabatic reactor. Ethanol vapor is fed to the reactor at 400 °C, and a conversion of
30% is obtained. Calculate the product Temperature.
CpSisto 48 có xio
7
+2.38 X10 T
05 x 10-872
C₂H5OH(v)→ CH3CHO(v) + H₂(g)
Solution
REACTOR
100 mol C₂H5OH(v)
400°C
C₂H50H (V), CH³ CHO (V), H₂
a
70 mol C₂H5OH(v)
30 mol CH3CHO(v)
30 mol H₂(g)
Tad (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The