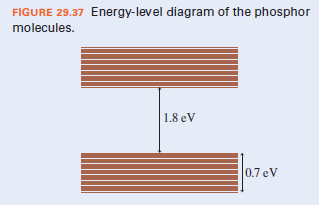
FIGURE 29.37 Energy-level diagram of the phosphor molecules. 1.8 eV 0.7 eV
how an ordinary incandescent bulb works: Current passes through a filament, heating it until it glows white hot. But such bulbs are very
inefficient, giving off only a few watts of visible light for every 100 W of electric power supplied to the bulb. Most of the power is, instead, converted into thermal energy. This is why, in recent years, incandescent lighting has given way to other types of lighting. The compact fluorescent
lamp has become a symbol of energy efficiency. These bulbs are about four times more efficient than incandescent bulbs at transforming electric energy into visible light. Inside the glass tube of a fluorescent bulb is a very small amount of mercury, which is in the form of a vapor when the bulb is on. Producing visible light occurs by a three-step process. First, a voltage of about 100 V is applied between electrodes at each end of the tube. This imparts kinetic energy to free electrons in the vapor, causing them to slam into mercury atoms and excite them by collisional excitation. Second, the excited atoms then jump to lower-energy states, emitting UV photons. Finally, the UV photons strike a phosphor that coats the inside of the tube,
causing it to fluoresce with visible light. This is the light you see.
a. A mercury atom, after being collisionally excited by an electron, emits a photon with a wavelength of 185 nm in a quan- tum jump back to the ground state. If the electron starts from rest, what minimum distance must it travel to gain enough kinetic energy to cause this excitation? The 60-cm-long tube has 120 V applied between its ends.
b. After being collisionally excited, atoms sometimes emit two
photons by jumping first from a high energy level to an intermediate level, giving off one photon, and then from this intermediate level to the ground state, giving off a second photon. An atom is excited to a state that is 7.79 eV above the ground state. It emits a 254-nm-wavelength photon and then a second photon. What is the wavelength of the second photon?
c. The energy-level diagram of the molecules in the phosphor is as shown. After excitation by UV photons, what range of wavelengths can the phosphor emit by fluorescence?
Trending now
This is a popular solution!
Step by step
Solved in 4 steps
