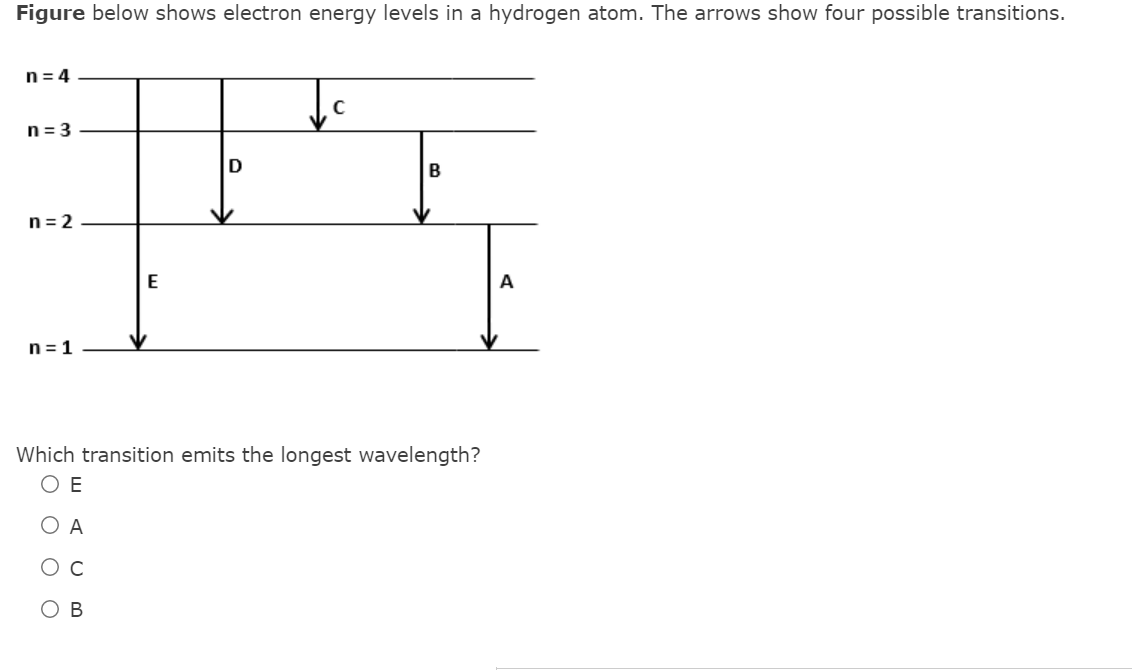
Figure below shows electron energy levels in a hydrogen atom. The arrows show four possible transitions. n= 4 n = 3 D B n= 2 E n = 1
Q: The Wanamaker Pipe Organ in Macy's Center City department store in Philadelphia is the largest worki...
A: Given Length of the pipe L=9.75m speed of sound v=345m/s
Q: A light source shines light consisting of two wavelengths, à, = 540 nm (green) and 2, = 450 nm (blue...
A: Given:
Q: Pr4. A vertical spring of stiffness k is atta- ched to the top of a block of mass m. At t = 0 the to...
A: Given that, The stiffness of the vertical spring = k Mass of the block = m The constant velocity of ...
Q: Please good handwriting and clear ideas explaining the process! 4E Solve the highlighted question ,...
A: e)E=ul'Au is energy densityE=12B2μ0×N2πr×AE=12μ0NI2πr2μ0×N2πr×Aafter putting of the values,E=976×10-...
Q: a) By considering a rigid body rotating about the axis O with an angular velocity (w) and accelerati...
A: The distance from location of the centre of gravity is XG. The moment of inertia I=mXG2 The linear v...
Q: A crate with mass 32.5 kg initially at rest on a warehouse flooris acted on by a net horizontal forc...
A: S=ut+1/2at2
Q: 11) What is a lodestone used for?
A: NOTE: As per Bartleby Guidelines only one part has to be solved at a time. Kindly upload other parts...
Q: 10 7 what is the change in electric potential moving from point P1 (P1, P1, z1) to point P2 (p2, 2. ...
A:
Q: 10. If DS1 shorted, what is the voltage across L-N if a multi-tester set at 600V DC range?
A: Given:- This instrument is used to measure the various parameters. The properties of el...
Q: A 27m3 CUBICAL TANK IS FULL OF TWO DIFFERENT FLUIDS OF EQUAL PARTS. AN OBJECT WAS THROWN AND WAS PAR...
A: The specific gravity of an object is the ratio of density of that object to the density of liquid in...
Q: Use the loop rule to determine the voltage at a with respect to b. The boxes can be sources or load...
A: Concept used: Kirchoff's law of voltage is used around the loop to calculate the voltage.
Q: A neutron in a nuclear reactor makes an elastic, head-on collision with the nucleus of a carbon atom...
A: Given
Q: Suppose you would like to introduce an interstitial or large substitutional atom into the crystal ne...
A: Where would the atom fit more easily above or below. given, figure
Q: 2. A Half-bridge inverter with resistive load and capacitive elements is given in the figure. Vb = 7...
A: Given: Vb=72 VC1=C2=240 μFR1=14 Ωf=120 Hz
Q: Teacher asked For any vector quantity, be sure to always include the magnitude and direction The ene...
A: (a) Workdone(W) by friction force. (b) What speed will the rock have by the time the skip let's go o...
Q: The figure blow shows the kinetic energy of a simple pendulum versus its angle θ from the vertical. ...
A: Given that a pendulum of mass m=0.2kg oscillates with maximum kinetic energy as given by the plot b...
Q: Two organ pipes, open at one end but closed at the other, are each 1.14 m long. One is now lengthene...
A: Beats are produced when two sound waves of same frequency superimpose while travelling through a med...
Q: A frictionless disk of mass 0.50 kg is moving in a straight line across an air table at a speed of 2...
A: Since the disk is moving on a frictionless surface, so it moves with a uniform velocity when it hits...
Q: Initially block of mass M is at rest on a frictionless floor and the spring is in relaxed condition ...
A: Initially block of mass M is at rest on a friction less floor and the spring is in relaxed condition...
Q: A uniform plane wave in air has the following expression E;(x, z) = ay. 5. e¯j(3ax+4nz) is incidence...
A: For a particular conductor, ET=HT=0 holds for transmitted waves. The reflected wave propagates in -a...
Q: Two pulses are shown moving towards each other. The pulses will meet and interact soon. Rank from g...
A:
Q: The current in a 20.0-mH inductor changes with time as i = 2.00t2 – 8.00t, where i is in amperes and...
A: Given data: Inductance of the inductor L=20.0 mH. Current i=2.00t2-8.00t
Q: Coulomb's Law
A:
Q: a) Give a statement of Gauss' Law in its integral form.
A: Gauss's law is used to find the electric field in any given region. Gauss' law states that the elect...
Q: An astronaut on Mars (mass 6.42 * 10^23 kg, radius3.39 * 10^6 m) launches a probe straight upward fr...
A: Given: Mass of the mars, M = 6.42 X 1023 Kg radius of the mars, R = 3.39 X 106 meter initial velo...
Q: 4. A student performs magnetic force experiment by using current balance setup below. A magnet of 46...
A: Given: The mass of the magnet is, m=46 g×10-3 kg1 g=0.046 kg. The length of the wire is, L=46 cm×10...
Q: Please good handwriting and clear ideas explaining the process! 1B Solve the highlighted question ,...
A: Given, Current=I Magnitude with magnetic field= Bo The magnetic field is parallel to side C.
Q: A wheel rotates from rest with constant angular acceleration. If it rotates through 8.00 revolutions...
A: Given that t = 2.50 sec, = 8.00 rev = 8.00x2xπ rad, initial angular velocity, ωi = 0 rad/sec By ...
Q: Q.4. CLO-1 Find the instantaneous velocity of the particle described in given Figure at the followin...
A: Given that: The position time graph is
Q: dison e horizontal plane. The pilot's location is defined by t ') and 0 = 2/n(sin t), where r, 60, a...
A: Given: To find the components of the radial and the transverse in which force exerts and the figure ...
Q: A coiled telephone cord forms a spiral with 90.0 turns, a diameter of 1.30 cm, and an unstretched le...
A: Given that,Nymber of turns (N) = 90Diameter of coiled telephone (D) = 1.30 cmRadius of of coiled tel...
Q: A conducting disk with radius a, thickness h, and resistivity p is inside a solenoid of circular cro...
A:
Q: A block rests on a frictionless surface and is attached to the end of a spring. The other end of th...
A: We know that the time period is inversely proportional to the frequency. here is the given diagram...
Q: In a potentiometer arrangement, a cell of emf 1.25 V gives a balance point at 35.0 cm length of the ...
A:
Q: A diver rotates once per second while in midair. If she pulls her legs in, reducing her rotational i...
A:
Q: A satellite 575 km above the earth’s surface transmits sinusoidal electromagnetic waves of frequency...
A: Given,
Q: An airplane flies northwest for 250 mi and then west for 150 mi. Find the resultant (or sum) displac...
A: Given:- 1. graph will be:
Q: A circular loop with radius 3 placed on the plane 2x + y + 2z = -8 has current equal to 2 A. What is...
A: Given, 2x+y+2z=-8 2x+y+2z+8=0 I=2A radius =3 we know,
Q: a) Explain the working principle of Kater’s Pendulum and it function in different systems.
A: A Kater's pendulum may be a reversible free swinging pendulum invented by British physicist and mili...
Q: Subject: Dynamics of Rigid Bodies
A: Given condition: x(0)=0
Q: i need the answer quickly
A: Given, Length of rod is, L Mass of rod is M, and θ is the angle from vertical (shown in figure) now...
Q: Q2:- find Iç, VcE 9v 31.1ka 3v 5602
A: 3=Vbe + Ie *560 since Vbe is not given we will take its value as 0 solving for Ie we get Ie=3/560 A...
Q: A watermelon is dropped from the edge of the roof of a building and falls to the ground. You are sta...
A: Let, v be the velocity of the watermelon when it is spotted first. The formula to calculate the heig...
Q: Help with number 3
A: A shunt circuit is defined together where all components are connected between an equivalent set of ...
Q: Assume a temperature of 300 K and fi nd the wavelength of the photon necessary to cause an electron t...
A: Given; Temperature=300 K Formula used:
Q: At a certain distance from a point charge, the potential andelectric-field magnitude due to that cha...
A:
Q: i need the answer quickly
A: The given lagrangian is L=12mx2-12ω2x2+αx4+βx2˙xω, α and β are constants in this equation, and there...
Q: A wave travels along a stretched horizontal rope. The vertical distance from crest to trough for thi...
A:
Q: You connect a 10.0 MΩ resistor in series with a 3.20 mFcapacitor and a battery with emf 9.00 V. Befo...
A:
Q: Bifocal lenses are prescribed for a patient, the components having focal length of 40cm & -300cm...
A: Given, Focal Length,f1=40cm=40×10-2mFocal Length,f2=-300cm=-300×10-2m Bifocal eyeglass lenses contai...
Step by step
Solved in 2 steps with 4 images
