Find the air fuel ratio for stoichiometric combustion of Ethene by volume. (26.19/1) 2. Find the air fuel ratio for stoichiometric combustion of Butane by volume. (30.95/1). Calculate the % carbon dioxide present in the dry flue gas if 30% excess air is used. (10.6%) 3. Find the air fuel ratio for stoichiometric combustion of Propane by volume. (23.81/1). Calculate the % oxygen present in the dry flue gas if 20% excess air is used. (3.8%) 1. A gaseous fuel contains by volume: 5% CO2, 40% H2, 40% CH4, 15% N2 Determine the stoichiometric air and the % content of each dry product. (4.76 m2, 89.7%, N2 10.3% CO2)
Find the air fuel ratio for stoichiometric combustion of Ethene by volume. (26.19/1) 2. Find the air fuel ratio for stoichiometric combustion of Butane by volume. (30.95/1). Calculate the % carbon dioxide present in the dry flue gas if 30% excess air is used. (10.6%) 3. Find the air fuel ratio for stoichiometric combustion of Propane by volume. (23.81/1). Calculate the % oxygen present in the dry flue gas if 20% excess air is used. (3.8%) 1. A gaseous fuel contains by volume: 5% CO2, 40% H2, 40% CH4, 15% N2 Determine the stoichiometric air and the % content of each dry product. (4.76 m2, 89.7%, N2 10.3% CO2)
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter39: Residential Energy Auditing
Section: Chapter Questions
Problem 15RQ: When performing a combustion efficiency test, digital flue gas will measure what four...
Related questions
Question
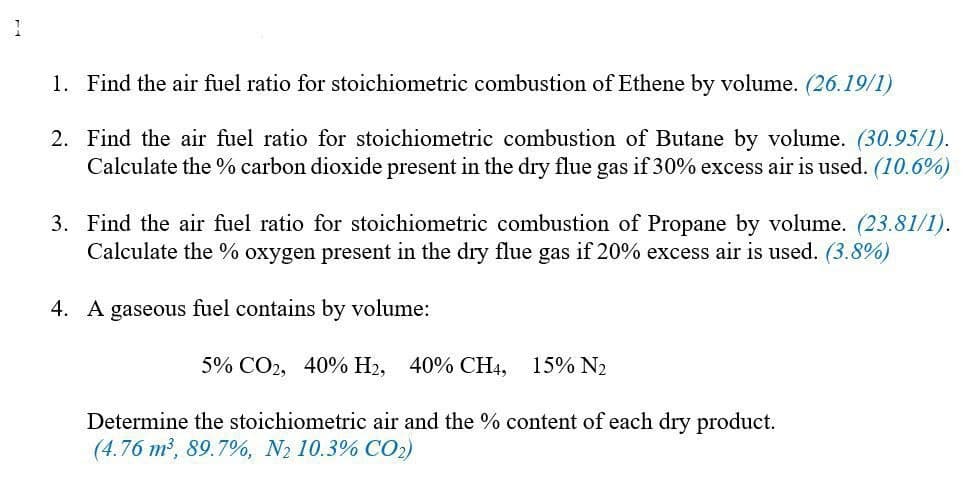
Transcribed Image Text:1. Find the air fuel ratio for stoichiometric combustion of Ethene by volume. (26.19/1)
2. Find the air fuel ratio for stoichiometric combustion of Butane by volume. (30.95/1).
Calculate the % carbon dioxide present in the dry flue gas if 30% excess air is used. (10.6%)
3. Find the air fuel ratio for stoichiometric combustion of Propane by volume. (23.81/1).
Calculate the % oxygen present in the dry flue gas if 20% excess air is used. (3.8%)
4. A gaseous fuel contains by volume:
5% CO2, 40% H2, 40% CH4,
15% N2
Determine the stoichiometric air and the % content of each dry product.
(4.76 m2, 89.7%, N2 10.3% CO2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning