For a K*- CF ion pair, attractive and repulsive energies E, and ER, respectively, depend on the distance between the ions r, according to 5.8 x 10-6 ER 1.436 For these expressions, energies are expressed in electron volts per K*- Cl- pair, and r is the distance in nanometers. a) If the net energy Ex is just the sum of the two expressions above: EN = E, + Eg , Find the values of r, and E,? OIf curves of E , E, and Ey are plotted in given figure, compare the calculated values of ro and Eo with that from the graph. -0.28 am E,--4.6 eV 9.00 0 19 120 c.30 040 262 0.70 Bonding Energy, eV
For a K*- CF ion pair, attractive and repulsive energies E, and ER, respectively, depend on the distance between the ions r, according to 5.8 x 10-6 ER 1.436 For these expressions, energies are expressed in electron volts per K*- Cl- pair, and r is the distance in nanometers. a) If the net energy Ex is just the sum of the two expressions above: EN = E, + Eg , Find the values of r, and E,? OIf curves of E , E, and Ey are plotted in given figure, compare the calculated values of ro and Eo with that from the graph. -0.28 am E,--4.6 eV 9.00 0 19 120 c.30 040 262 0.70 Bonding Energy, eV
Related questions
Question
100%
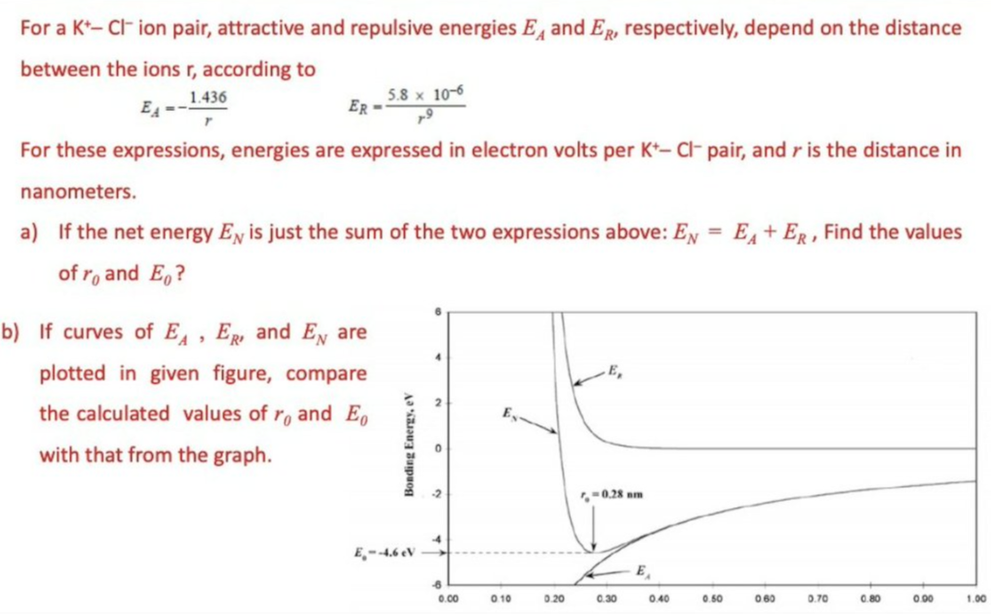
Transcribed Image Text:For a K*- CF ion pair, attractive and repulsive energies E, and ER, respectively, depend on the distance
between the ions r, according to
1.436
E4 --
5.8 x 10-6
ER =
For these expressions, energies are expressed in electron volts per K*- Cl- pair, and r is the distance in
nanometers.
a) If the net energy EN is just the sum of the two expressions above: EN = E+ ER, Find the values
of r, and E,?
b) If curves of E,, ER and EN are
plotted in given figure, compare
E,
the calculated values of ro and E,
with that from the graph.
-0.28 nm
E,-4.6 eV
0.00
0 10
0.20
0.30
0.40
0.50
060
0.70
0.80
00
1.00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images
