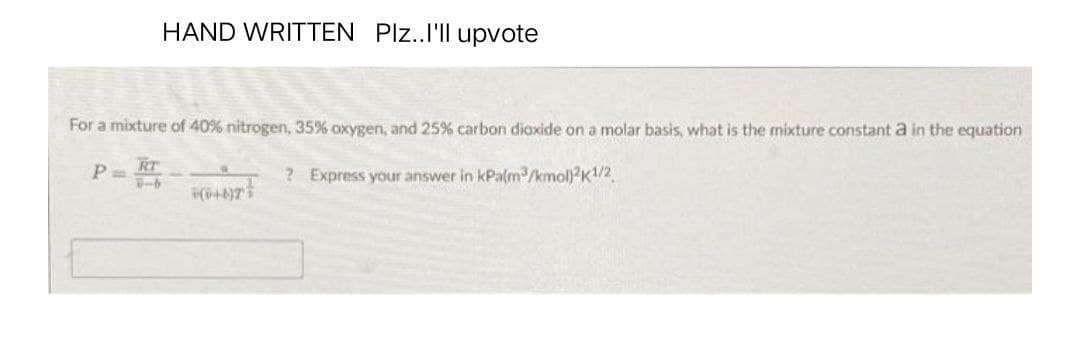
For a mixture of 40% nitrogen, 35% oxygen, and 25% carbon dioxide on a molar basis, what is the mixture constant a in the equation P= RT ? Express your answer in kPa(m³/kmol)2K1/2 HOT!
Q: A2.1-m-long, 0.2-cm-diameter electrical wire extends across a room that is maintained at 20°C. Heat…
A: When an electric current is passed through a conductor due to the resistance heat is generated. The…
Q: ) Transmission of ultrasound waves through a specific tissue : will change it Frequency intensity…
A: The transmission of ultrasound waves through different tissues with different Speed. In increasing…
Q: For known boundary conditions and fluid properties, a general compressible flow problem has five…
A: The unknowns for a compressible flow problem are pressure, the three components of velocity, and the…
Q: e The potential applied external to a conductor is given by the expression P Р -pline + 1 д д…
A:
Q: A hand-cranked generator can be connected to a number of light bulbs via a switch. Is it easier to…
A: If the switch is closed or open during the turning of the handle of the generator there will be…
Q: Consider a person whose exposed surface area is 1.7 m2, emissivity is 0.5, and surface temperature…
A: Heat flow takes place from a region of higher temperature to a region of lower temperature. In…
Q: 1. For the discussed problem: loop of current with radius R at xz plane. (a) Sweep point P location…
A:
Q: 1. (20 pts) An 2 particle has rest energy 1672 MeV and mean lifetime 8.2 x 10-¹1 s. It is created…
A: Calculate the total energy of the particle Ω- by using the relativistic energy equation as given by…
Q: A child applies a push on his toy car causing it to move horizontally. He controls his applied push…
A:
Q: 5. If 528 kJ of heat are added to 2.5 kg of helium at constant volume process when the initial…
A:
Q: While Uranium-235 is commonly used in nuclear power plants, the isotope U-238 is more commonly found…
A:
Q: SA: A solar cell operating at a temperature of T=300K has the following specifications T= 5x10° Sec,…
A:
Q: Given that the initial length of the metal is 2 meters and the final length is 2.03. meters. The…
A: Given: Original Length of metal, Lo=2 m Final length of metal, L=2.03 m Change in temperature, ∆T=88…
Q: The function of density value for a liquid is given as p = Kh+po, where K is a dimensional constant…
A:
Q: . What is the electric potential 2.5 x 10-15 m away from a proton (+e)?
A: Given r= 2.5×(10) ^(-15)meter Proton charge e= 1.6×(10) ^(-19)Coulomb
Q: Q. ADJ Express the force F in Cartesian vector from if Point B is located 2m along the rode 6m 6m D…
A: Given: Length of AB = 2 m Coordinate of point A = (0,0,6) Coordinate of point C = (-3,4,0)…
Q: *61. ssm A cube is located with one corner situated at the origin of an x, y, z, coordinate system.…
A:
Q: (20 pts) Quasars are among the most distant objects in the universe and are moving away from us at…
A: Solution:- Given,Z=λ'-λsourceλsource = Z=λ'λsource-1…
Q: (b) A diesel engine has a compression ratio of 18 and a cut off ratio of 2.4. At the beginning of…
A:
Q: A parallel-plate capacitor illustrated in the figure has a plate area of 10 cm2 that are separated…
A: Two capacitors connected in parallel have their equivalent capacitance given as Ceq=C1+C2 And the…
Q: Q2/ In mammographic examinations, the breast is compressed between two plates. Answer the following…
A: Solution:- a) The geometric unsharpness is decreased by compression because the…
Q: Consider the following unattached spring system. m₁ = 1, C₁ = 14, m₂ = 7 Write the stiffness matrix…
A: “As per the policy we are allowed to answer only 3 subparts at a time, I am providing the same.…
Q: 65 20 8 Q3: Mention the basic drawing terms and the modification terms needed to draw the figure…
A:
Q: A wire loop of area A=0.12m² is placed in a uniform magnetic field of strength B=0.2T so that the…
A:
Q: A radioactive element has rate of disintegration 10,000 disintegrations per minute at a particular…
A: Given-Rate of disintegration=10000/mintime=4 minutesNew rate of disintegration=2500/minTo find-decay…
Q: How to make a lever change color of a cube in unity? Lever should toggle if it reaches up or down
A:
Q: 04 16 small compact sphere with a uniform mass density rolls without slipping on a roller coaster…
A: Given: The mass of the sphere is m=40 g=0.04 kg. The radius of the sphere is r=10 mm=0.01 m. The…
Q: 50 degrees 2. Two vectors, F1 = 150 N and F2= 200 N, have directions as shown in the figure below.…
A: Given, Two vectors in xy plane
Q: What will be the Schwarzschild radius of Earth?
A: Schwarzschild radius also known as the gravitational radius is the minimum radius below which causes…
Q: number of wire coils on magnetic force? Magnet #1 Magnetic force strength: ))) Magnet #2 Magnetic…
A: Disclaimer: “Since you have asked multiple question, we will solve the first question for you. If…
Q: A temperature controller, designed to work in a steam envi- ronment, involves a bimetallic strip…
A:
Q: The temperatures shown in the figure have been determined via 2-dimensional finite difference…
A:
Q: Does the rubber band follow Hooke’s law? • Is there evidence that the rubber band follows Hooke’s…
A:
Q: 81. mmh Find the magnitude and the direction of the current in the 2.0- resistor in the drawing. 1.0…
A: Draw the circuit diagram as below.
Q: 11. Two coils P and Q are kept near each other. When no current flows through coil P and current…
A: To find-Magnetic flux=?Given-Two coils P and QEmf in the coil P=ep=15 mVdIdtQ=10ASCurrent in the…
Q: A long D anrying nd 1
A: Magnetic fields are generated by the flow of current in a conductor. The magnetic field generated…
Q: If a 1.5 m long string on the same wave machine has a tension of 360 N, and the wave speed is 450…
A: Given, Length L = 1.5 m Tension T = 360 N Wave speed v = 450 m/s…
Q: Two small nonconducting spheres have a total charge of Q-Q+Q₁-95.0 μC, QiQ When placed 22.0 cm…
A:
Q: 1. Using the configuration shown in Figure 1, show that: mλ = dsin(0), (1) where A is the wavelength…
A: 01) Given:
Q: A voltage phasor diagram of a series LC circuit is given below. Find the approximates values of: 1.…
A:
Q: For safety in climbing, a mountaineer uses a nylon rope that is 65 m long and 1.4 cm in diameter.…
A:
Q: A radioactive element has rate of disintegration 10,000 disintegrations per minute at a particular…
A: Given-Rate of disintegration=10000/mintime=4 minutesNew rate of disintegration=2500/minTo find-decay…
Q: The wooden pile shown in the figure has a diameter of 115 mm and is subjected to a load of P = 80…
A: It is given that, Diameter = 115 mm = 0.115m Load, P = 80 kN Frictional resistance, w= 3.94 kN/m…
Q: Consider the following arrangement: String Steel cube A and B are weighing machine based on springs.…
A: From Archimedes principle the apparent weight of a body is recorded when it is placed in fluid.…
Q: The function of density value for a liquid is given as p = Kh+ p₁, where K is a dimensional constant…
A: Solution:-Given thatρ=Kh+ρ∘
Q: 11.18 A 15,000-N crane .. Figure E11.18 Bricks pivots around a friction-free axle at its base and is…
A: a) Find the moments around the axle. Take clockwise as positive. For the system to be in balance…
Q: Find the relation between backwards finite difference and average operator.
A: Given : backward finite difference operator and average operator. Our task : find relationship…
Q: A ball with a radius of R_sphere and a mass of M begins at rest down a ramp without slipping…
A: Given:radius of the ball, Rspherefor simplicity, let Rsphere =Rslet radius of the loop be, Rloop =…
Q: Q1: Consider the diameter of carbon fiber is 7 um and the diameter of glass fiber is 16 µm. (a)…
A:
Q: The radioactive nuclide 33 Bi decays into 34 Po. 83 84 (a) Write the nuclear reaction for the decay…
A: Given that:-After Beta decay Bi83215 converted to Po84215 Bi83215⇌β- decay Po84215
Step by step
Solved in 2 steps
