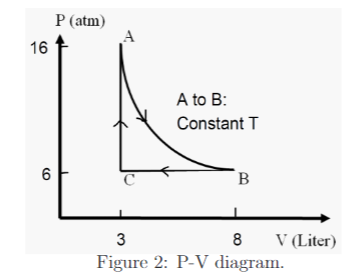
Four (4) moles of helium gas undergo the following cyclic process (A→B→C→A). If 1 atm = 1.0 ×105 Pa, and the total internal energy of such an ideal gas equals 3/2 nRT, where n, R and T are the number of moles of the gas, the universal gas constant and temperature in kelvin, respectively, (a) what is the total amount of work done on the gas in one cycle? (b) what is the amount of heat involved in one cycle? (c) what is the change in entropy of the gas from A to B in terms of R, the universal gas constant?
Four (4) moles of helium gas undergo the following cyclic process (A→B→C→A). If 1 atm = 1.0 ×105 Pa, and the total internal energy of such an ideal gas equals 3/2 nRT, where n, R and T are the number of moles of the gas, the universal gas constant and temperature in kelvin, respectively, (a) what is the total amount of work done on the gas in one cycle? (b) what is the amount of heat involved in one cycle? (c) what is the change in entropy of the gas from A to B in terms of R, the universal gas constant?
Related questions
Question
Four (4) moles of helium gas undergo the following cyclic process (A→B→C→A).
If 1 atm = 1.0 ×105 Pa, and the total internal energy of such an ideal gas equals 3/2 nRT,
where n, R and T are the number of moles of the gas, the universal gas constant and
temperature in kelvin, respectively,
(a) what is the total amount of work done on the gas in one cycle?
(b) what is the amount of heat involved in one cycle?
(c) what is the change in entropy of the gas from A to B in terms of R, the
universal gas constant?
Transcribed Image Text:P (atm)
16
A to B:
Constant T
6.
B
V (Liter)
Figure 2: P-V diagram.
3
8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images
