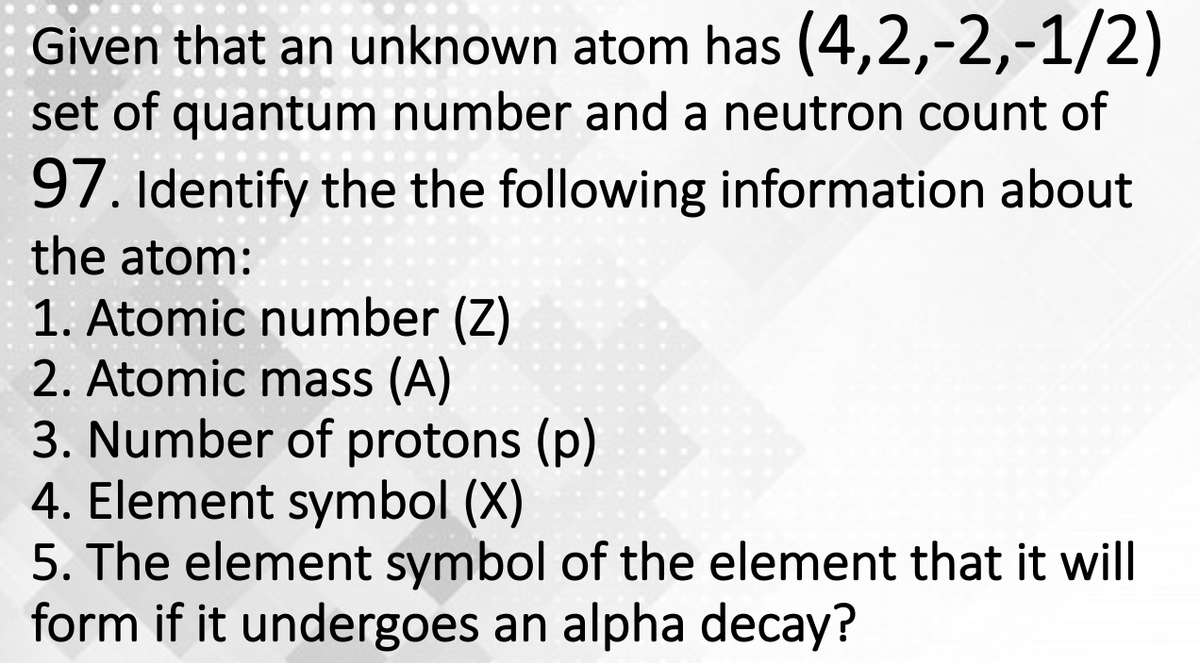
Given that an unknown atom has (4,2,-2,-1/2) set of quantum number and a neutron count of 97. Identify the the following information about the atom: 1. Atomic number (Z) 2. Atomic mass (A) 3. Number of protons (p)
Q: Use your expressions to find the aphelion speed for Comet Halloy Express
A:
Q: 25 g of dry ice (solid CO2) is placed in a container which has an initial volume 2.00 x 10^4 cm^3,…
A: Given that:-Dry ice= 25 gVolume of container, V=2×104 cm3V=2×10-2 m3Temperature, T=0°then, T=273…
Q: Calculate the energy of the photon having a wavelength of 500 nm. O point
A: Photons are packets of energy. Each photon will have a certain amount of energy defined by, E=hv…
Q: Ex. 9: The horizontal telegraph wire 100 m long, oriented along magnetic east west falls freely…
A: To find-Induced emf (e)=?Given-Length of wire (l)=100 mVelocity (V)=20 cm/s =0.2…
Q: The cirouit shown in the figure contains two batteries, each with an emf and an internal resistance,…
A:
Q: (b) The Lagrangian function of a mechanical system with one degree of freedom is ma – U(z), L =…
A:
Q: What happens to a white dwarf when a normal star dumps mass to a white dwarf?
A: The white dwarfs are stars that have burned up all of the hydrogen they once used as nuclear fuel.
Q: How much energy is required to propel a 60.0 KG person to 45.2 cm vertically? What is the person's…
A:
Q: nk starts to flow at a rate (q.). F« dz ntial equation () that best de de
A: Here, mass generated = mass consumed = 0.
Q: Determine the magnitude of the required force. In order to make the rod of the (Figure 1) move to…
A:
Q: A planet with earth Ilike magnetic field can be considered as a bar magnet. Suppose, we have such a…
A: You have posted a question with more than 3 subparts. As per our policy, we will solve first three…
Q: $6
A: Given that:-Power, P=F.v equation 01Expressions for the forceF=mgSinθ+Ffricction Equation…
Q: The adorable cat below asks, "What is currently the best theory for the nature of Dark Matter?" What…
A: Dark Matter: Dark matter is a component of the universe whose gravitational attraction, rather than…
Q: What is the constant acceleration of the system? What is the tension in the cord connecting blocks A…
A: Given, mA=1 kgmB=2 kgmc=3 kgθ=300
Q: What is the magnitude of the electric field 90.30 cm above a 0.00054C charge? • Do NOT include units…
A:
Q: A 49 K2 resistor is connected with a 12 mF with a battery of 7 V. How much voltage will be dropped…
A:
Q: A child of mass 30 kg is playing on a maypole swing in a playground. The length of rope is x = 2.8 m…
A:
Q: 4. A rock contains the radioactive isotope K – 40 that decays with a half-life of 1.3 billion years…
A: Given A rock contains the radioactive isotopes of K-40 and radioactive isotopes of Rb-87 half-life…
Q: 1. What is the angular momentum of a particle of mass m, moving in a circle of radius r with an…
A:
Q: The Giant Metrewave Radio Telescope (GMRT) near Pune has several antennas spread over a region of…
A: When two close objects at a large distance are observed they might seem like a single source. The…
Q: a A unisaxm Siexible sting is ends Sixead at x-A is acded on y a Voriable extermal Soxce such that…
A: It is known that, Laplace transform in general form is written as, F(s)=∫0+∞f(t)·e-stdt It is given…
Q: Reflection by thin layers. In the figure, light is incident perpendicularly on a thin layer of…
A: Given data n1=1.59n2=1.37n3=1.45L=416 Here, n represents the refractive indexes of different…
Q: How much energy is lost to a dissipative drag force if a 60-kg person falls at a constant speed for…
A: Given, m=60 kgh=15 m
Q: State the definition of the quasi-Fermi energy level.
A: The steady state condition for the electron and hole populations is described by separate fermi…
Q: in the arrangement shown in the figure below, an object of mass m = 4.0 kg hangs from a cord around…
A:
Q: 7) Where is the block located, relative to equilibrium, at a time 0.99 s after it is released? (if…
A: Given data : Block mass m =5kg Spring constant Kleft = 31 N/m Spring constant Kright = 56 N/m…
Q: no induced charge in the picture
A: The properties of charge ad its quantization are to be recalled.
Q: Q. 23 : Two particles of masses m, and mb and of the same charged are projected in a perpendicular…
A: To find-True statement Given-Mass of particle (a) is=maMass of particle (b)=mbRadius of path…
Q: How much energy is lost to a dissipative drag force if a 60-kg person falls at a constant speed for…
A:
Q: A muon is traveling at 0.996c. What is its momentum? (The mass of such a muon at rest in the…
A: Given that:Speed of muon, v=0.996cMass of muon is 107meme is mass of electron.
Q: growth rate of 0.2 in
A: Given as, N1=35 individuals,N2=1588 individuals.
Q: A plank of wood (p 600 kg/m) with dimensions (2 x 0.02 x 0.15) m is floating on water (p 1000 kg/m).…
A: Given that:Wood density, ρ1=600 kg/m3Stone density, ρ2=1000 kg/m3Wood volume, V1=2×0.02×0.15 m
Q: As measured by an observer on the earth, a spacecraft runway on earth has a length of 3200 m. What…
A: The observer at rest with respect to the earth measures the length of the runway to be 3200 m. This…
Q: 2. From the rules governing the use of quantum numbers, show that the K, L and M shells in an ator…
A: Given that the atom contains K,L and M shells.
Q: Z. R
A:
Q: Three point charges q are placed at the corners of an equilateral triangle. Another point charge -Q…
A:
Q: Whale and Kanmuro are two islands located at the Chimera archipelago. These two are separated by a…
A:
Q: Consider the Lagrangian function 1 L = m (x² + j² + ż²) + 16ý sin (x – t), where m is a positive…
A:
Q: Find a + b + c + d + e. ;0 <t< 플 ; 풀 St<ris: t ;a<t<27 The Fourier series of the function f(t) = (늘…
A: It is known that general form of Fourier series is, f(t)=a0+∑n=1∞ancos2nπL·t+∑n=1∞bnsin2nπL·t here,…
Q: r q2 to have a zero net force along the y-axis?
A:
Q: Light of wavelength 602 nm is incident normally on a diffraction grating. Two adjacent maxima occur…
A: Given: Incident wavelentgh, 602 nm sinθ=0.23sinθ=0.34 One-fourth maxima is missing To find: For slit…
Q: . Supply the missing steps on equation below B Hod х-dy 2a (x² + y² ³/2 47 xVx² +a²
A:
Q: The primary of a biansformere har, ho tins and norke loo uolt and lo0 wall; Fnd the mumbeu of lins…
A:
Q: ith work, potential ener r work.
A: The Relation between electric potential and work as, W=q∆V, ∆V is the potential difference.
Q: What is the power rating of a stove that receives 12.5 A of current and requires 150 V to function?…
A: Given that:- Current, I=12.5 A Potential V=150 V Power is given as: P=VI Equation…
Q: g) What is the period of the object? (with reference to the velocity in letter e) f) What is the…
A: Given an object with mass m=0.30kg is being whirled vertically using a rope of length r=1.0m Let R…
Q: 5. Three oscillators of equal mass m are coupled such that the potential energy of the system is…
A: Given: The potential energy of the system is U = 12 k1x12 +x32 + k2x22 + k3 x1x2 + x2x3 Where k3 =2…
Q: 8. Junction
A: The problem is based on the basic concept of current electricity. In an electric circuit, we use…
Q: A thin rod of mass m = 12 kg is constrained to rotate about an axis through its center, but there is…
A: Given : Mass, m=12 kg Angle, 17° Rod length, l=2.5 m ω=5 rad/s To find: Torque
Q: In a bipuism expt. the dislance uulual between the lwo images f the, slit is l5 mm 4 the clislance…
A: To find-Distance between 2nd and 8th dark fringe on same side of central band x8-x2=?Given-d=1.5…
Step by step
Solved in 2 steps
