Given the following reactions, find the standard cell potential. Anode: NO3- + 2H+ + e- --> NO2 + H2O Cathode: 2H+ + 2e- --> H2
Given the following reactions, find the standard cell potential. Anode: NO3- + 2H+ + e- --> NO2 + H2O Cathode: 2H+ + 2e- --> H2
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
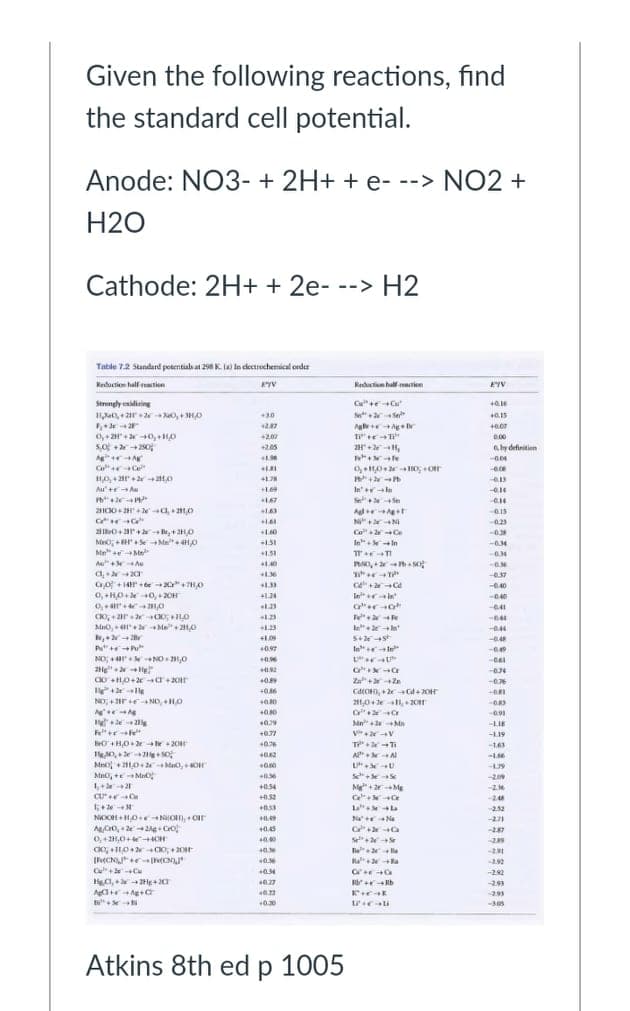
Transcribed Image Text:Given the following reactions, find
the standard cell potential.
Anode: NO3- + 2H+ + e- --> NO2 +
H2O
Cathode: 2H+ + 2e- --> H2
Table 72 Standard potentials at 298 K (a) in cdectrochemical oeder
Reduction half eaction
Reductiom half stien
ETV
Strengly eidising
C"+eC"
+30
SeaSe
0.15
+287
Ae Ag
+6.00
+200
+2.05
aby definitien
H0, +2 ato
In le
Se+ Se
Adr t
N Ni
Co Ce
In"+ In
Au+ A
167
163
100
131
-634
Mn Me
+1.51
-6.34
A + Au
1.40
-0.M
+1.36
-0.37
1.33
-C.40
0.+ HO 40,2OH
le rale
-0.40
0,r 1,0
-641
Te+ e
-044
.44
.
S+
te l
-0.4
0.97
NO NO ,0
-0.4
Za Ze
-0.76
NO, +r NO, +HO
211,04e 20
-0.83
G30
0.39
Mn e Mn
LIE
+0.77
-L19
BOHOr +20
07%
-163
0,+ so
Mno+2LO Ma, OH
062
A AI
-19
Muo, +e- Mno
+0.56
-209
+0.54
Me+ Me
-2.16
+0.52
-2.44
-2.32
Na Na
C Ca
0.45
-287
0,42H,0+ CH
40.00
Se Se
-299
-2.91
-2.92
+0.34
-2.92
0.27
-2.93
-2.93
+0.30
Atkins 8th edp 1005
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The