Given the work functions for some metals:- Aluminum=4.08eV, Beryllium=5.0eV, Cadmium=4.07eV, Calcium=2.9eV, Carbon=D4.81eV, Cesium=2.1eV, Cobalt=5.0eV, Copper=4.7eV, Gold=5.1eV, Iron=4.5eV, Lead=4.14eV, Magnesium=3.68€V, Mercury=4.5eV, Nickel=5.01eV, Niobium=4.3eV, Platinum=6.35eV, Potassium=2.3eV, Selenium=5.11eV, Silver=4.73eV, Sodium=2.28eV, Uranium=3.6eV, and Zinc= 4.3eV. If you shine light with a wavelength of 212 nm on Niobium, what is the kinetic energy of the electrons being emitted. Provide your answer in units of 10*19 J.
Given the work functions for some metals:- Aluminum=4.08eV, Beryllium=5.0eV, Cadmium=4.07eV, Calcium=2.9eV, Carbon=D4.81eV, Cesium=2.1eV, Cobalt=5.0eV, Copper=4.7eV, Gold=5.1eV, Iron=4.5eV, Lead=4.14eV, Magnesium=3.68€V, Mercury=4.5eV, Nickel=5.01eV, Niobium=4.3eV, Platinum=6.35eV, Potassium=2.3eV, Selenium=5.11eV, Silver=4.73eV, Sodium=2.28eV, Uranium=3.6eV, and Zinc= 4.3eV. If you shine light with a wavelength of 212 nm on Niobium, what is the kinetic energy of the electrons being emitted. Provide your answer in units of 10*19 J.
Related questions
Question
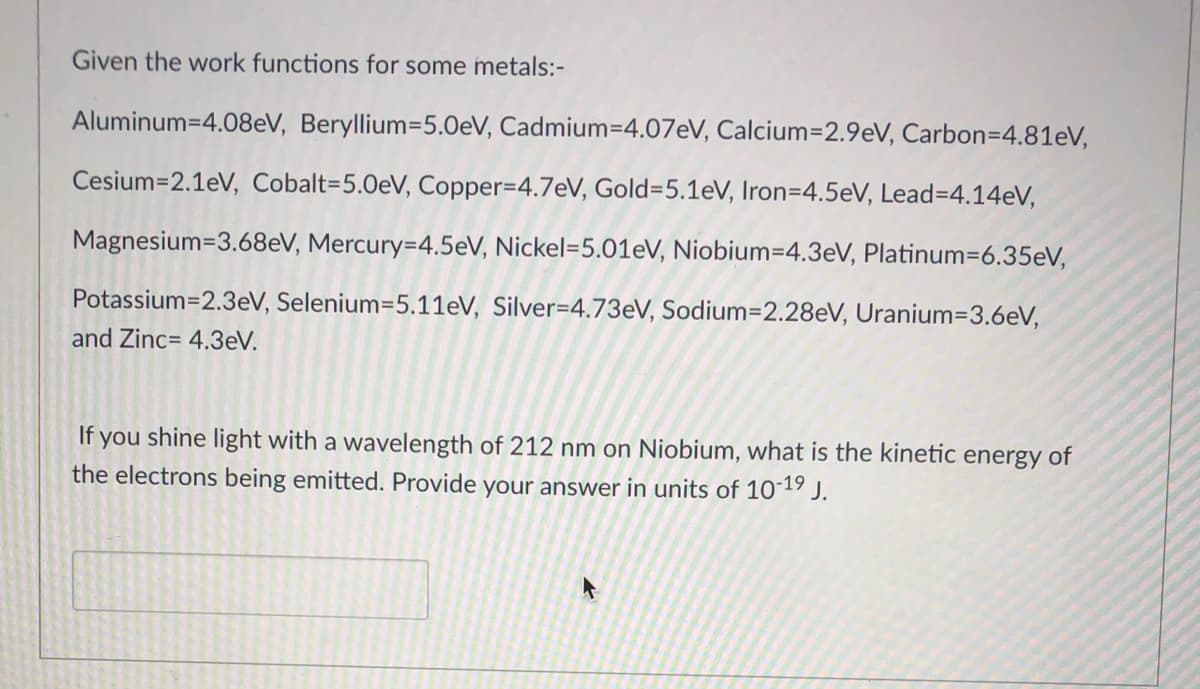
Transcribed Image Text:Given the work functions for some metals:-
Aluminum=4.08eV, Beryllium=5.0eV, Cadmium=4.07EV, Calcium=2.9eV, Carbon=4.81eV,
Cesium=2.1eV, Cobalt=5.0eV, Copper=4.7eV, Gold=5.1eV, Iron=4.5eV, Lead=4.14eV,
Magnesium=3.68eV, Mercury=4.5eV, Nickel=5.01eV, Niobium=4.3eV, Platinum=6.35eV,
Potassium=2.3eV, Selenium=5.11eV, Silver=4.73EV, Sodium=2.28eV, Uranium=3.6eV,
and Zinc= 4.3eV.
If you shine light with a wavelength of 212 nm on Niobium, what is the kinetic energy of
the electrons being emitted. Provide your answer in units of 1019 J.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
