Grape must with a specific temperature of 2.8 kJ / kg C and 85% water is concentrated in a single-stage evaporator to 60% dry matter and molasses is produced. Grape must enters the evaporator at a speed of 1500 kg / hour and at a temperature of 45 C. In the evaporator, saturated steam at a pressure of 170 kPa is used as a heater and the evaporation process is carried out under a vacuum of 0.75 atm. The total heat transfer coefficient of the evaporator is determined as 850 W / m2C. Boiling point and heat of dissolution variations are negligible. Calculate the amount of heating steam, steam economy and total heat transfer surface area accordingly.
Grape must with a specific temperature of 2.8 kJ / kg C and 85% water is concentrated in a single-stage evaporator to 60% dry matter and molasses is produced. Grape must enters the evaporator at a speed of 1500 kg / hour and at a temperature of 45 C. In the evaporator, saturated steam at a pressure of 170 kPa is used as a heater and the evaporation process is carried out under a vacuum of 0.75 atm. The total heat transfer coefficient of the evaporator is determined as 850 W / m2C. Boiling point and heat of dissolution variations are negligible. Calculate the amount of heating steam, steam economy and total heat transfer surface area accordingly.
Initial moisture content = 85 %
Final solute concentration = 60 %
Feed flow rate = 1500 kg/h
Feed temperature = 45 0C
Specific heat of feed = 2.8 kJ/kg∙ 0C
Saturated steam is used as the heating medium.
Pressure of saturated steam = 170 kPa
Overall heat transfer coefficient = 850 w/m2∙ 0C
Boling point and heat of dissolution are negligible.
Grape is concentrated in a single effect evaporator. In the evaporator process the solution is concentrated by removing the solvent (mostly water) by evaporation.
The block diagram is shown below:
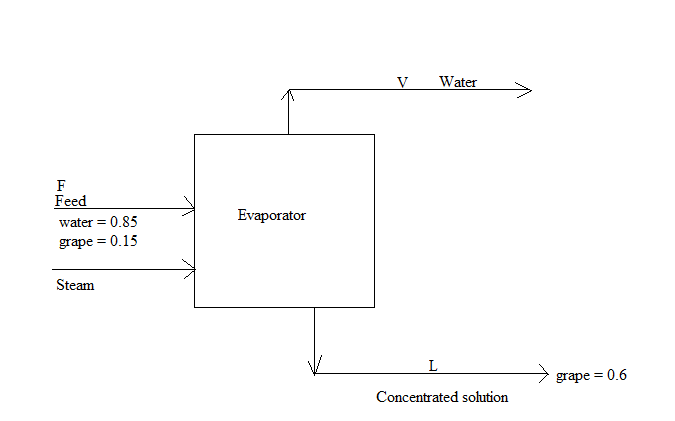
The flow rates of all the streams area calculated as follows:
Apply the overall balance
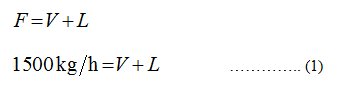
Where,
V represents the flow rate of water vapor.
L represents the floe rate of concentrated solution.
Apply the solute balance
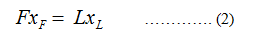
Where,
F represents the floe rate of feed.
xF represents the composition of solute in feed.
xL represents the composition of solute in the concentrated solution stream.
F = 1500 kg/h
xF = 0.15
xL = 0.6
Plug-in the values in equation (2)
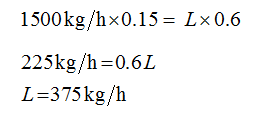
Plug-in the value of L in equation (1)
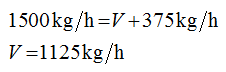
The amount of heating steam is calculated as follows:
Saturated steam is used as the heating medium.
Pressure of saturated steam = 170 kPa
From the steam table, the latent heat of condensation of steam at 170 kPa (388 K) is 2215.75 kJ/kg.
The process occurs at vacuum pressure of 0.75atm, the enthalpy of vaporization of water at 0.75 atm and 365 K (approximate) is 2275 kJ/kg.
Apply the enthalpy balance
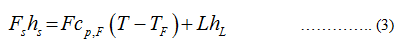
Where,
hs represents the enthalpy of condensation of steam.
Cp.F represents the specific heat of feed.
hL represents the enthalpy of vaporization of water.
T represents the temperature corresponding to the evaporator pressure.
TF represents the temperature of feed.
Plugin the values in equation (3)
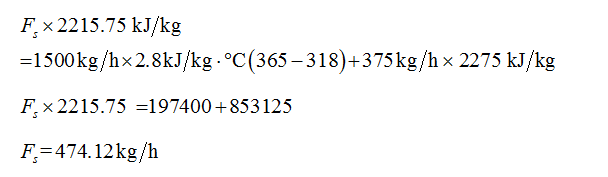
Step by step
Solved in 7 steps with 12 images









