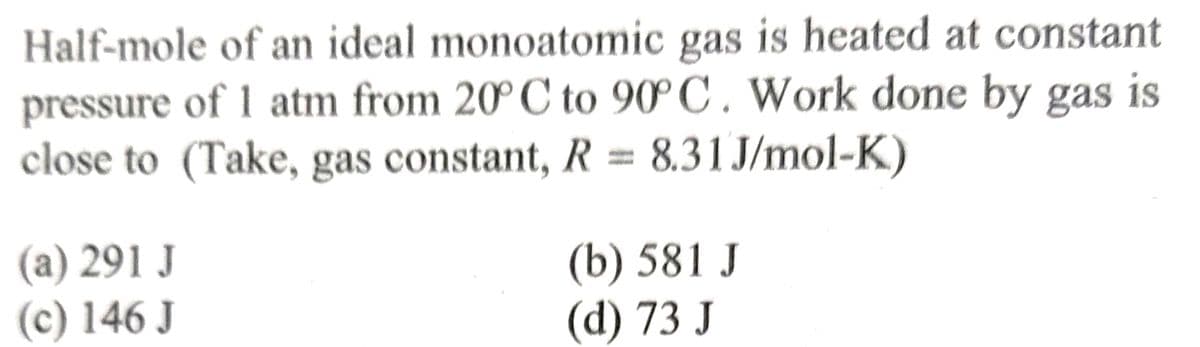
Half-mole of an ideal monoatomic gas is heated at constant pressure of 1 atm from 20° C to 90°C. Work done by gas is close to (Take, gas constant, R = 8.31J/mol-K) (a) 291 J (c) 146 J (b) 581 J (d) 73 J
Q: Let ƒ(x, y) = x² + 4y² and let C be the line segment from (0, 0) to (2, 2). You are going to compute…
A: Given: f(x,y)=x2+4y2 We need to find the line integral in two different ways.
Q: An alternating emf e = 100 sin. 314t is applied Selimeen terminals of an electric bulb whose Alament…
A: We need to compute-(i) RMS current (ii) Frequency of A.C.(iii) Period of A.C.The data given…
Q: Compare the acceleration of a motorcycle that accelerates from 80 km/h to 90 km/h with the…
A: We have to determine the comparison of acceleration of motor cycle with the acceleration of a…
Q: Review figure 7.7 in section 7.4. What is the approximate orbital radius of Jupiter? a. 20 AU b. 1…
A: Given: We have to find orbital radius of Jupiter
Q: The graph below shows the force needed to stretch a spring. What work is needed to stretch it from 5…
A:
Q: or to a 37 volt s eater' is applie w long will it ta
A: Given: Resistor is 1.6 ohm Voltage is 37 V Temperature is 37oC Volume of water is 40 mL
Q: DVD disc rotates from rest up to an angular velocity of 242.4 rad/s in a time of 0.912 s. (a)…
A: Disclaimer: “Since you have asked posted a question with multiple sub-parts, we will solve the first…
Q: 23. What is the current / in the circuit as shown in figure: (A) 2 A (C) IA (B) 1.2 A (D) 0.5 A 202…
A: Given data, Circuit diagram is given as, Equivalent resistance of the circuit is given by,…
Q: Fill in the blanks for the missing values in the table.
A:
Q: The incident voltage wave on a certain lossless transmission line for which Zo= 50 Q and vp = 2x108…
A: Given that output impedance (Z0)=50Ωvelocity vp=2×108m/sVoltage V+(z, t)=200 cos(ωt-πz)........(1)If…
Q: Having been in position for a long time, the switch in the circuit below is moved to position b at…
A: Here is explanation of the above problem.
Q: Assume that the universe consists of only two stars. At some time t2, the stars are separated by a…
A: The formula for gravitational potential energy is U=-Gm1m2r where U is the potential energy, G is…
Q: If unifoum copper cuire of length 5m & diameter 3x10³ m in extended by 10³m. Calculate the Encigy…
A: We need to compute-Energy stored in wire (E)=?The data given as-L=5 mr=d2=3×10-32=1.5×10-3 ml=10-3…
Q: Calculate A) the conservative force described by the potential energy function U(x,y,z) = ½ k (2x³…
A: We have given a potential energy U(x,y,z)=1/2k(2x3+5y2-3z4) We know that a conservative force can we…
Q: xx=41 Q1. Consider an extrinsic Silicon doped with Indium atoms at a concentration of N₁ = 4x10¹ cm³…
A:
Q: Consider Newton's second Law F = må . Solve for m.
A:
Q: 65-kg astronaut pushes against the inside back wall of a 2000-kg spaceship and moves towards the…
A: Solution: Applying the momentum conservation of the system. Assuming the astronaut and spaceship are…
Q: 3. Electron's trajectory. Consider an electron with an initial velocity of magnitude to directed at…
A: Disclaimer: “Since you have asked posted a question with multiple sub-parts, we will solve the first…
Q: An interstellar cloud fragment 0.2 light-year in diameter is rotating at a rate of one revolution…
A:
Q: Calculate the period of Mercury around the Sun using the Earth's period and orbital radius as a…
A: Given data: Orbital Radius of Earth, rES= 1.50 x 1011m Orbital Radius of Mercury, rMS = 5.79 x…
Q: The first excited state of the one-dimensional harmonic oscillator has eigenfunction y(x) =…
A: Have a look dear
Q: Two rocket ships approach Earth from opposite directions, each with a speed of 0.85c relative to…
A: Given that, One rocket ship is travelling toward the world at a speed of 0.85 c, while another…
Q: A long solenoid has 98 turns/cm and carries current i. An electron moves within the solenoid in a…
A: The speed of the electron is v=0.0375 c where c is the speed of light. v=0.0375×2.998×108…
Q: A rectangular slab (length 2.5 cm, width 1.5 cm, thickness 5.0 mm) of an unknown semiconductor with…
A:
Q: A student (m = 69 kg) falls freely from rest and strikes the ground. During the collision with the…
A:
Q: A stream of water strikes a stationary turbine blade horizontally, as the drawing illustrates. The…
A:
Q: Create a problem for which the solution is: (0.02 kg)(300 m/s)−(10 N)(0.4 m/s)=(0.02 kg)(100 m/s)…
A: We are given the equation of solution. We need to formulate a problem. We see in problem that the…
Q: Hi, I am in need of help on this physics question: Devise an experiment to measure the spring…
A: We are given the spring. The spring is extended to certain length. We need to measure the spring…
Q: A current I is flowing along a cylindrical conductor of radius a is made of material with…
A: Given,The current I flowing along a cylindrical conductor of radius a is made ofmaterial with…
Q: The acceleration of gravity at the surface of a planet of radius 6,106 km is 10.7 m/s². What is the…
A: The acceleration due to gravity in m/s2 at 295 km from the planet's surface. given, Radius of…
Q: The photoelectric current in a photoelectric cell can be reduced to zero by a stopping potential of…
A: We need to compute-Maximum kinetic energy of photoeectrons(KEmax)=?The data given as-Stopping…
Q: A particle at t₁ = -2.0 s is at x₁ = 4.3 cm and at t₂ = 4.5 s is at x₂ = 8.5 cm. What is its average…
A: We have to determine the average velocity from the given data. Given that, t1=-2.0 sx1=4.3 cmt2=4.5…
Q: Y = 3 cos 100x (2t - x), the value of λ is
A:
Q: A projectile of mass mp is traveling at a constant velocity up toward a stationary disk of mass M…
A: Given,The mass of the projectile= mp The velocity of the projectile= v→0iMass of the disk = MRadius…
Q: What will happen to the speed of the conductor when the conductor is shorted? E
A: Given that There is a straight conductor A force F applied on it. There is a magnetic field, B is…
Q: DYNAMICS OF RIGID BODIES UPVOTE WILL BE GIVEN.PLEASE WRITE THE COMPLETE SOLUTIONS CORRECTLY AND…
A:
Q: The escape velocity from the earth's surface is 11.2 km/s. If the mass of the Jupiter is 318 times…
A: We need to compute-Escape velocity (Ve2)=?The data given as-Ve1=11.2 km/sMass of jupiterMass of…
Q: 1. A dam is in the shape of an isosceles trapezoid. The dam is 120 feet wide at the top and 60 feet…
A: From figure we need to apply pythagorous theorem on ∆ABEAB2=BE2+AE2BE2=AB2-AE2BE2=842-642BE=54.50…
Q: The radius of a uniform solid sphere is measured to be (6.50 ± 0.20) cm, and its mass is measured to…
A: Given that-Radius, r=6.50±0.20 cmMass, M=1.85±0.02 kgWe know that,Density, ρ=M43πr3
Q: 1 [Select] J Consider the scale above. Making a measurement with this scale will give [Select]…
A: We will answer this question by analysing the divisions in the given scale.
Q: You travel from point A to point B in a car moving at a constant speed of 70 km/h. Then you travel…
A: In the hypothetical scenario, we drive a car from point A to point B at a constant speed of 70…
Q: Please refer to picture. A hydraulic jack is used to lift a car in a mechanic's shop. The mechanic…
A:
Q: The moment of inertia of a disc about an axis passing through its centre and perpendicular to its…
A:
Q: Quartz has hexagonal 32 point group symmetry. Its unit cell dimensions are a = 4.914 Å and c = 5.405…
A: We have given that, quartz has hexagonal 32 point group symmetry. It unit cell dimensions are a =…
Q: plane ngm distance must you set the focus of the camera lens in order to snap a sharp picture of the…
A: Lens focal length defines the point where parallel light rays meet. Getting a sharp handheld shot…
Q: 220 V is supplied across 1200 windings of the primary coil of the autotransformer. If 1650 windings…
A: Given Number of turns in primary coil is Np=1650 Number of turns in…
Q: The law of reflection is quite useful for mirrors and other flat, shiny surfaces. (This sort of…
A: (a) From Snell's law, when a ray of light is incident at angle θa from normal, it makes same angle…
Q: I E V(x) Vo X=0 II Q/Solve the Schrödinger equation for regions x0 to calculate the reflection…
A:
Q: A disk of radius 20 cm is has a charge of 2x10- 12, uniformly distributed along the edge of the…
A: Given, Radius, r=20cmr=0.2m Charge, Q=2×10-12C Angular velocity, ω=12rad/s The acceleration is,…
Q: (a) How much energy is stored in a 10.2 mH inductor carrying a 1.15 A current? (b) How much current…
A: Given: The given values are, The energy value is, ε=1J L=10.2 mHL=10.2×10-3H To find the current…
Step by step
Solved in 2 steps
