Helium gas has a volume of 250 mL at 273Kat 1.0 atm. What will be the final pressure ( P2 ) if the volume is reduced to 100 mL at 318K?
Helium gas has a volume of 250 mL at 273Kat 1.0 atm. What will be the final pressure ( P2 ) if the volume is reduced to 100 mL at 318K?
Engineering Fundamentals: An Introduction to Engineering (MindTap Course List)
5th Edition
ISBN:9781305084766
Author:Saeed Moaveni
Publisher:Saeed Moaveni
Chapter10: Force And Force-related Variables In Engineering
Section: Chapter Questions
Problem 26P: SAE 30 oil is contained in a cylinder with inside diameter of 1 in. and length of 1 ft. What is the...
Related questions
Question
Solve the given problem using the equation of Combined gas law.
1. Helium gas has a volume of 250 mL at 273Kat 1.0 atm. What will be the final pressure ( P2 ) if the volume
is reduced to 100 mL at 318K?
2. The volume of a gas at 270C and 700.0 mmHg is 600mL.What is the final volume ( V2 ) of the gas at -200C
and 500.0 mmHg?
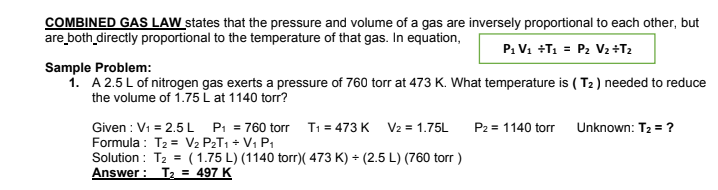
Transcribed Image Text:COMBINED GAS LAW states that the pressure and volume of a gas are inversely proportional to each other, but
are both_directly proportional to the temperature of that gas. In equation,
P: V1 +T1 = P2 V2+T2
Sample Problem:
1. A 2.5L of nitrogen gas exerts a pressure of 760 torr at 473 K. What temperature is ( T2 ) needed to reduce
the volume of 1.75 L at 1140 torr?
Given : V1 = 2.5L P: = 760 torr T1 = 473 K V2 = 1.75L
Formula : T2 = V2 P2T1 + V1 P1
Solution : T2 = (1.75 L) (1140 torr)( 473 K) + (2.5 L) (760 torr )
Answer : T2 = 497 K
P2 = 1140 torr Unknown: T2 = ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Engineering Fundamentals: An Introduction to Engi…
Civil Engineering
ISBN:
9781305084766
Author:
Saeed Moaveni
Publisher:
Cengage Learning

Engineering Fundamentals: An Introduction to Engi…
Civil Engineering
ISBN:
9781305084766
Author:
Saeed Moaveni
Publisher:
Cengage Learning