How much energy (in Joules) is required to convert 126 grams of ice at -26 °C to liquid water at 40 °C? (The specific heat of ice = 2.10 J/g C; the specific heat of water = 4.184 J/g°C; the heat of fusion = 333 J/g°C). (The answer should be reported as the aboslute value--no sign is required.) Submit Question
How much energy (in Joules) is required to convert 126 grams of ice at -26 °C to liquid water at 40 °C? (The specific heat of ice = 2.10 J/g C; the specific heat of water = 4.184 J/g°C; the heat of fusion = 333 J/g°C). (The answer should be reported as the aboslute value--no sign is required.) Submit Question
Algebra & Trigonometry with Analytic Geometry
13th Edition
ISBN:9781133382119
Author:Swokowski
Publisher:Swokowski
Chapter2: Equations And Inequalities
Section2.1: Equations
Problem 74E
Related questions
Question
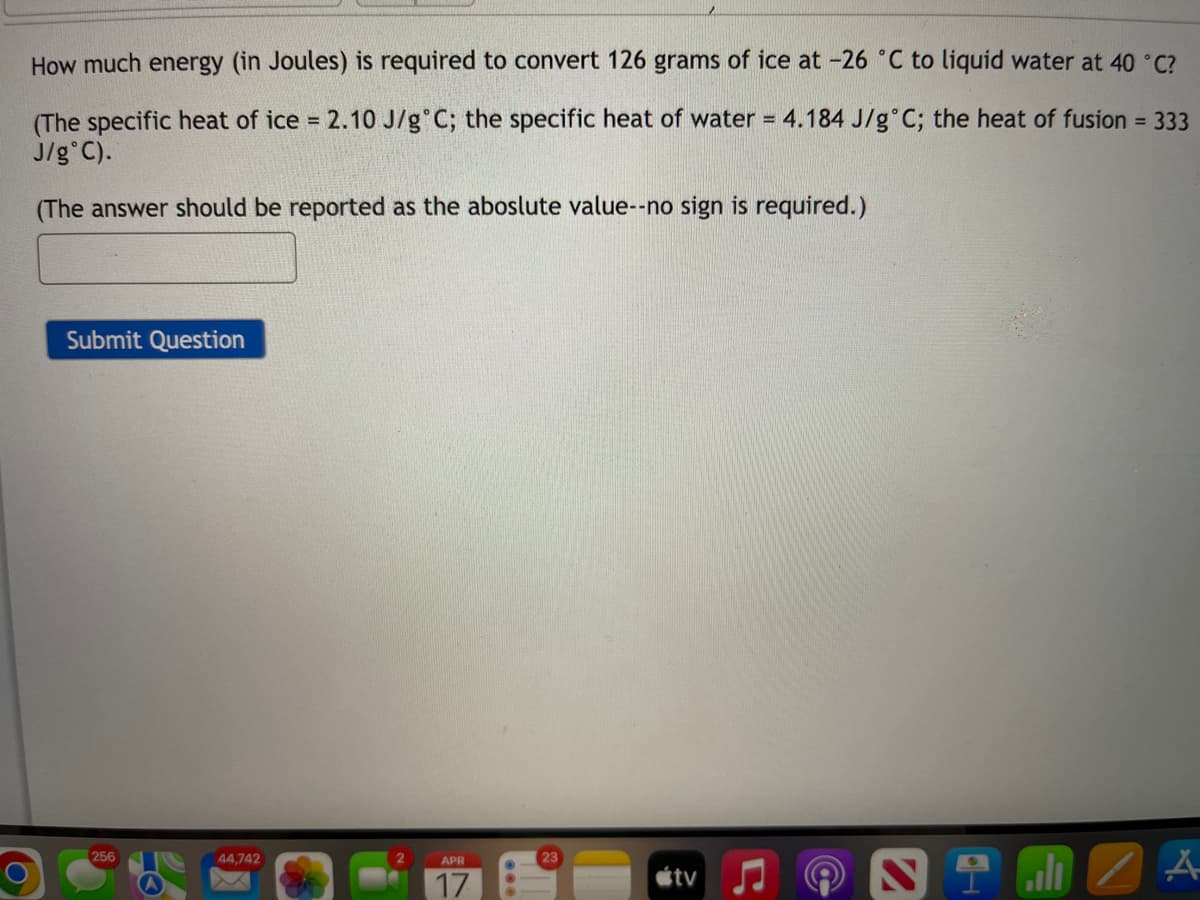
Transcribed Image Text:How much energy (in Joules) is required to convert 126 grams of ice at -26 °C to liquid water at 40 °C?
(The specific heat of ice = 2.10 J/g°C; the specific heat of water = 4.184 J/g°C; the heat of fusion = 333
J/g°C).
(The answer should be reported as the aboslute value--no sign is required.)
Submit Question
256
44,742
APR
23
17
stv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning

Algebra: Structure And Method, Book 1
Algebra
ISBN:
9780395977224
Author:
Richard G. Brown, Mary P. Dolciani, Robert H. Sorgenfrey, William L. Cole
Publisher:
McDougal Littell