(i) An isothermal expansion till it reches volume 2V, and heat Q flows into the gas (ii) An isobaric compression back to the original volume V (iii) An isochoric increase in pressure till the original pressure P is regained. The efficiency of this cycle can be expressed as 0 (a) == (c) == 4Q-2RT 4Q+3RT 4Q-2RT 40+ RT (b) == (d) E= 40+2RT 4Q-3RT 4Q+2RT 40+ RT
(i) An isothermal expansion till it reches volume 2V, and heat Q flows into the gas (ii) An isobaric compression back to the original volume V (iii) An isochoric increase in pressure till the original pressure P is regained. The efficiency of this cycle can be expressed as 0 (a) == (c) == 4Q-2RT 4Q+3RT 4Q-2RT 40+ RT (b) == (d) E= 40+2RT 4Q-3RT 4Q+2RT 40+ RT
Related questions
Question
Transcribed Image Text:()
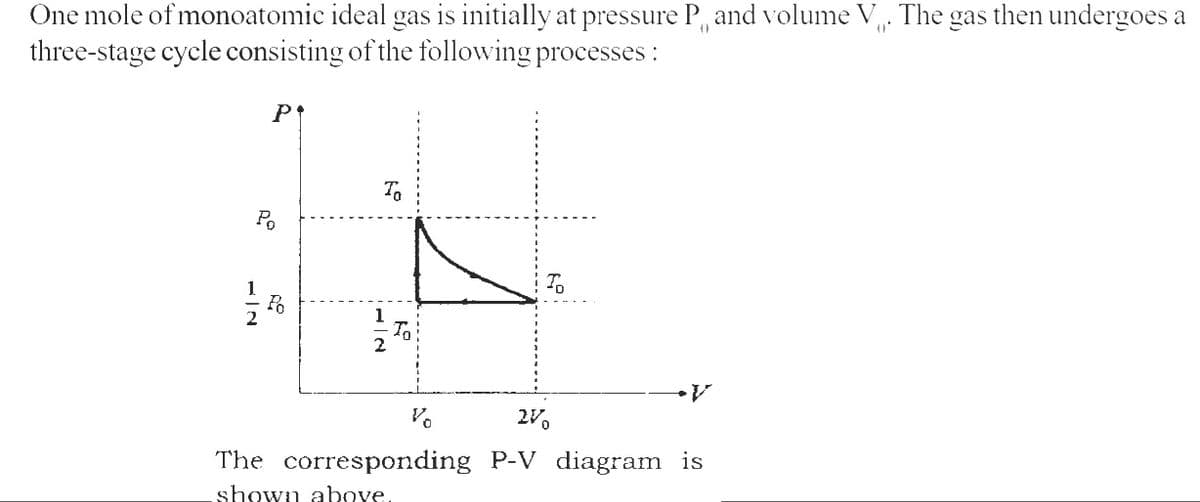
One mole of monoatomic ideal gas is initially at pressure P and volume V. The gas then undergoes a
three-stage cycle consisting of the following processes:
-2
P↑
Po
To
1
2
To
Vo
To
2V0
The corresponding P-V diagram is
shown above.

Transcribed Image Text:(i) An isothermal expansion till it reches volume 2V, and heat Q flows into the gas
(ii) An isobaric compression back to the original volume V
0
(iii) An isochoric increase in pressure till the original pressure P is regained.
The efficiency of this cycle can be expressed as
(a) ==
(c) ==
4Q-2RT
4Q+3RT
40-2RT
4Q+RT
(b) ==
(d) ==
40+2RT
4Q-3RT
40+2RT
40+ RT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images
