I Review | Constants | Perio A glass tube contains 2 x 10" atoms, some of which are in the ground state and some of which are excited. (Figure 1) shows the populations for the atoms' three energy levels. Part A Is it possible for these atoms to be a laser? If so, on which transition would laser action occur? O 3+ 2 O 3+1 O 2+1 O Not possible Figure 1 of 1
I Review | Constants | Perio A glass tube contains 2 x 10" atoms, some of which are in the ground state and some of which are excited. (Figure 1) shows the populations for the atoms' three energy levels. Part A Is it possible for these atoms to be a laser? If so, on which transition would laser action occur? O 3+ 2 O 3+1 O 2+1 O Not possible Figure 1 of 1
Related questions
Question
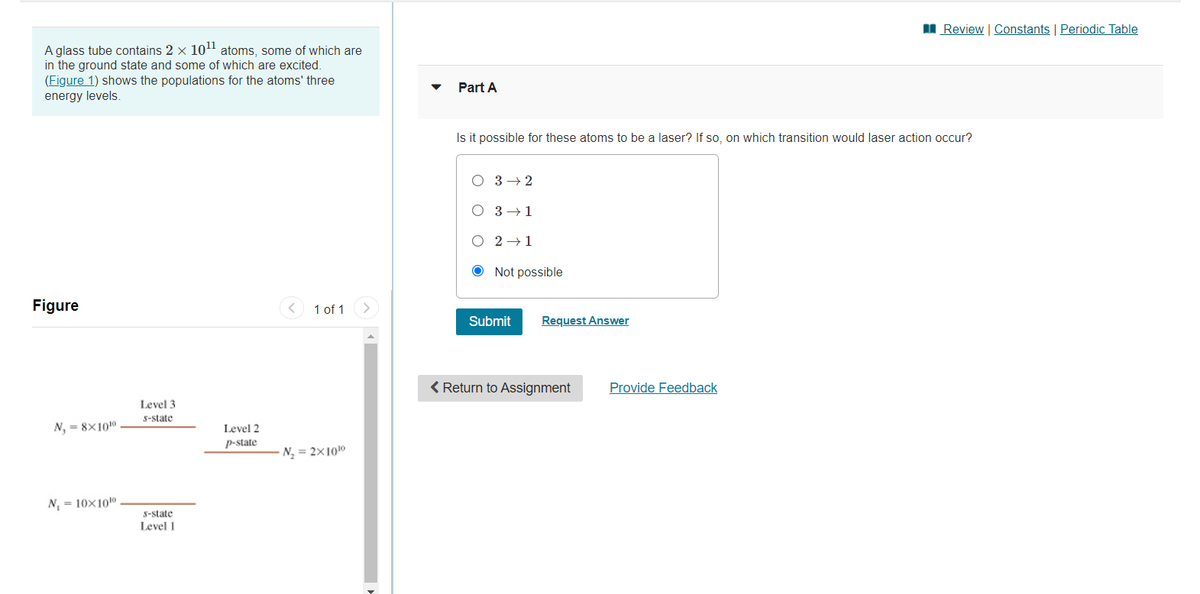
A glass tube contains 2×10112×1011 atoms, some of which are in the ground state and some of which are excited. (Figure 1) shows the populations for the atoms' three energy levels. Is it possible for these atoms to be a laser? If so, on which transition would laser action occur?
Transcribed Image Text:I Review Constants | Periodic Table
A glass tube contains 2 x 10" atoms, some of which are
in the ground state and some of which are excited.
(Figure 1) shows the populations for the atoms' three
Part A
energy levels.
Is it possible for these atoms to be a laser? If so, on which transition would laser action occur?
O 3→ 2
O 3+1
O 2→1
Not possible
Figure
1 of 1
Submit
Request Answer
< Return to Assignment
Provide Feedback
Level 3
S-state
N, = 8×100
Level 2
p-state
N = 2x1010
N, = 10x10
S-state
Level 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps
