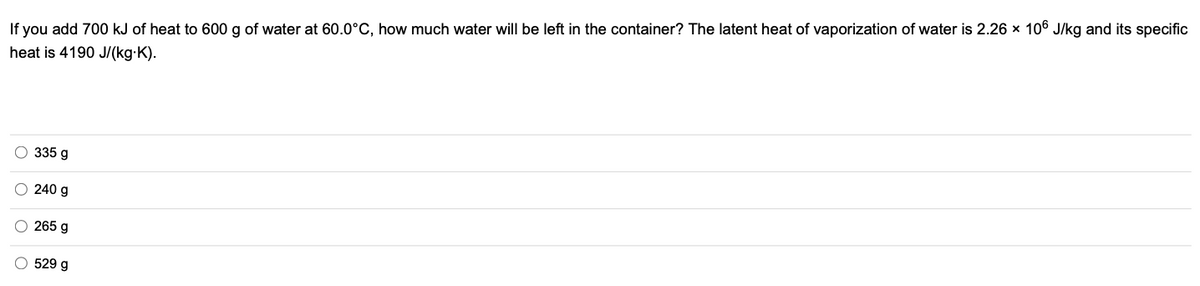
If you add 700 kJ of heat to 600 g of water at 60.0°C, how much water will be left in the container? The latent heat of vaporization of water is 2.26 × 106 J/kg and its specific heat is 4190 J/(kg-K). O 335 g O 240 g O 265 g O E00 o oo
Q: Subject Test Note: - You are attempting question 5 out of 12 Calculate the tunneling probability whe...
A: The kinetic energy of the particle is given as, E=0.2 MeV The barrier height is given as, Uo=20 MeV ...
Q: 6cose Problem #5: Given the ekctric fiekd intensity vector E = R OSO R? (V/m), R? A) Find the ekctri...
A:
Q: 1. Solve the Schrodinger equation for a particle of mass, m, in a box. The box is modeled as an infi...
A: 1) Given: Length of the box is L. Potential inside the box is V0 Calculation: The schematic diagram ...
Q: Good density is obtained with a SID of 36 inches at 19 mAs. The next X-ray on the patient requires t...
A:
Q: An object of mass 8.0 kg is attached to an ideal massless spring and llowed to hang in the Earth's g...
A: Here first we will calculate spring constant using Hook's law. Then we will use frequency formula
Q: Let D = y?z°a + 2xyz*ay + 3xy² z²a, nC/m2 be 2,3. ,2. %3| the electric flux density in free space. F...
A: To solve the problem, we will write expression for electric flux and substitute the given values. Th...
Q: Simplify the expression(1+cotx)(1-cotx)-csc square x
A: The mathematical expression is given as f(x)=1+cot x1-cot x- cosec2xThis mathematical expression is...
Q: (a) What is an electric charge? (b) Write and comment on Coulomb's Law and Its relatlonshlp to the e...
A: These are simple concepts. Note:- as per our guidelines I can provide 3 answers per session. Please ...
Q: .A ring of radius R having a uniformly distributed charge Q is fixed in a horizontal plane. A bead o...
A: in the following problem, the gravitational force acting downwards , balances the coloumbic repulsio...
Q: 3. You are driving in Europe and you see that the posted is 90 seconds per hour (km/hr.). How many m...
A: Given,Speed limit =90km/hr1km =0.62miles90km=0.62×90=55.8 milesin miles per hour =55.8m/h
Q: Consider air as being composed of a mixture of ideal gases. a. How many moles of air are present in...
A:
Q: Find the angle between the angular momentum vector L and the z axis for all possible orientations wh...
A:
Q: 3. The students in this class performed an experiment to determine the acceleration of a ball due to...
A:
Q: Q. 12: The distance between centre of the earth and moon is 384000 m. if the mass of the earth is 6 ...
A: To find-Speed of moon=?Given- Orbital radius of moon (r)=384000 km=3.84×105 kmMass of earth (M)=6×10...
Q: Statistical Description of Particle Systems Review the clasic harmonic oscillator consisting of the ...
A: Given Review the classic harmonic oscillator consisting of the mass m and the spring constant k has ...
Q: Car A is traveling at the constant speed of 46 km/h as it rounds the circular curve of 310-m radius ...
A: The way is correct, please do verify later for calculation correction (if any)
Q: a) If the temporal time evolution of the state vector ) governed by Schrodinger equation, ihw.) = H\...
A:
Q: 5a. Determine the centroid of the shape belows YA 2" 7" 3" 7" 3"
A: Solution: The centre point of the two shapes is drawn as follows
Q: The guy cables AB and AC are attached to the top of the transmission tower. The tension in cable AB ...
A:
Q: During the collision ball X comes to rest and block Y starts moving towards point B. from position A...
A: Given: The length of the inextensible string is l = 1.5 m The string makes an angle at the point A i...
Q: A system consists of two concentric cylindrical conductive shells of length L >>> d (a, b, c, d defi...
A: The two concentric cylindrical shells are made of conducting materials. The inner shell has a total...
Q: E Assume an electron is initially at the ground state of a l-D infinite square well and is exposed t...
A: Here we will use time dependent perturbation theory. Let us first find out the matrix coefficient fo...
Q: Electric Flow Exercise Consider the enclosed cylindrical gaussian surface attached. Suppose that the...
A: Concept: According to Gauss' law If the volume within an arbitrary closed mathematical surface conta...
Q: O. 14 : Work done in blowing a soap bubble of diameter 2 cm, is (S.T. = 3 x 10-² N/m) (a) 7.54 x 10 ...
A: To find-Work done (W)=?Given-Diameter of soap bubble D=2 cmRadius =D2=22=1 cmRadius(r)=1×10-2 mT=3×1...
Q: A machine part is vibrating along the x-axis in simple harmonic motion with a period of 0.27 s and a...
A:
Q: s due to both electrons
A: It is known that the current density as, δ=IωtI=δωtI=nqvωt
Q: Q. 37 : What value of speed must be given to a projectile so as to launch it form earth's surface at...
A: To find-VC=?Given-h=14RR=radius of earth
Q: Good density is obtained with a SID of 36 inches at 19 mAs. The next X-ray on the patient requires t...
A: For constant density , mAs and SID are related by the proportional square relation mAs1mAs2=SID1SID2...
Q: There is a liquid mixture at 20 ° C of 40% acetic acid and 60% by mass of water. Calculate the densi...
A: The following data are given: Mass percentage of acetic acid, macid=40% Mass percentage of water, mw...
Q: Solve for the resultant of à = 3.0N, 150°, B = 2.0N,100° and č = 1.0N,50° using component method. %3...
A: Since we only answer up to one question we will answer the first question only. Please resubmit the ...
Q: 3.4. The path that the particle follows, its trajectory, is something that particle physicists study...
A:
Q: make questions and discussions about forced damped oscillations
A:
Q: 3. Refer to the image at the right. True or False? A = B 4. Refer to the image at the right. True or...
A: 3.) The two vectors A and B have the same magnitude but are opposite in direction. Thus, they are no...
Q: The Sun produces energy at a rate of 4.00 x 1028 W by the fusion of hydrogen. If the Sun is 90.0% hy...
A: Given that,E=4×1026 JouleE=mc2 Equation 1where, c=3×108m/sfrom equation 1,m=Ec2=4×1026 Joule3×108m...
Q: 1. A force of 69N is needed to start an 6.9kg box moving across a horizontal surface. Calculate the ...
A: Given, A box is moving in frictiotional surface.
Q: 1. An archer is 10 meters away from the target. If the arrow is at chest high upon release which is ...
A: This problem is based on projectile motion, where the initial velocity is to be evaluated. Height fr...
Q: 1. The following data is available for an undoped semiconductor: n, = 1010 /cc, T= 300 K, N. = 3 x 1...
A:
Q: Draw the electric field lines. 2) what electrode was connected to the +ve terminal?
A: The electric field lines at the edges will curve as they repeal each other but inside region they wi...
Q: make questions and discussions about forced damped oscillations
A:
Q: the system in Fig. 1.1 is at 20 ° C. If the pressure at point A is 1900 lbf / ft2, determine the pre...
A: Given: Pressure at point A is 1900 lbf / ft2 The diagram is drawn as,
Q: d D, and the integration path taken. tial of point B with respect to point D if ID and the integrati...
A: Given,
Q: SCIENCE 10 Activity 1-Learning Task 1 (page 8) Instruction: Study the activity. Follow the procedure...
A: Recording Station Time difference of P-wave and S-wave (Seconds) Distance of the Epicenter from s...
Q: 6sine Problem #5: Given the electric field intensity vector E = R R? 6cose (V/m), R? A) Find the eec...
A:
Q: You step on a weighing scale, and it tells you mass 63 kilograms. How many pounds does this represen...
A:
Q: 6sine Problem #5: Given the electric field intensity vector E = R R? 6cose (V/m), R? A) Find the eec...
A:
Q: (10r") b. Given that D=(10r²) ar (c/m²) in cylindrical coordinates, evaluate both sides of the diver...
A:
Q: A square surface measures 3.2 mm on each side. It is immersed in a uniform electric field with magni...
A:
Q: he 0. 15 : The masses of two plane ratio 1 : 2. Their radii are in the ratio 1 : 2. The acceleration...
A: To find-g1g2=?Given-Ratio of masses=m1m2=1:2Ratio of radii=r1r2=1:2
Q: (2a) Answer the following (i) Y N Pf W Why? "ii) Y N PI w Why? (ii) Y N Pf W
A: Note: Since we only answer up to 3 sub-parts, we’ll answer the first 3. Please resubmit the question...
Q: 14 1 A v2 v3 121. 15 V. i3
A:
Step by step
Solved in 3 steps with 2 images
