Ignoring the fine structure splitting, hyperfine structure splitting, etc., such that energy levels depend only on the principal quantum number n, how many distinct states of singly-ionized helium (Z = 2) have energy E = -13.6 eV? Write out all the quantum numbers (n, l, me, ms) describing each distinct state. (Recall that the ground state energy of hydrogen is E₁ -13.6 eV, and singly-ionized helium may be treated as a hydrogen-like atom.)
Ignoring the fine structure splitting, hyperfine structure splitting, etc., such that energy levels depend only on the principal quantum number n, how many distinct states of singly-ionized helium (Z = 2) have energy E = -13.6 eV? Write out all the quantum numbers (n, l, me, ms) describing each distinct state. (Recall that the ground state energy of hydrogen is E₁ -13.6 eV, and singly-ionized helium may be treated as a hydrogen-like atom.)
Related questions
Question
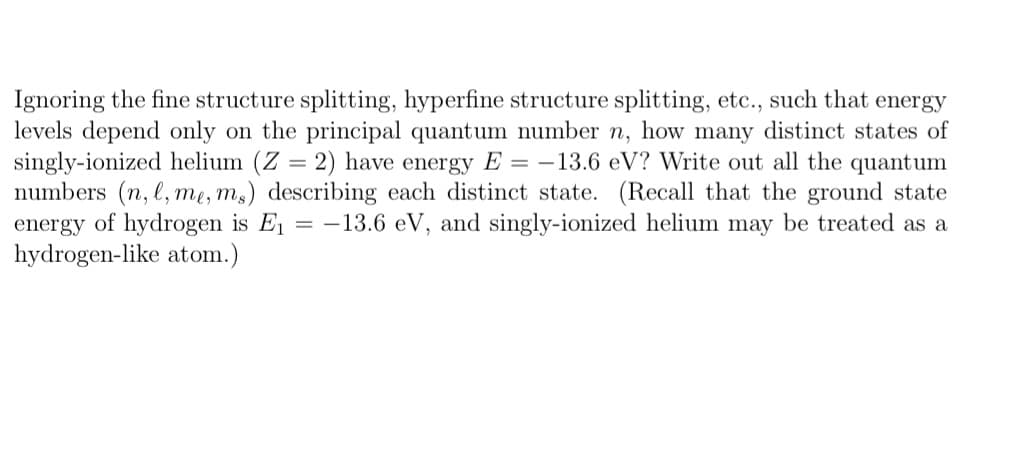
Transcribed Image Text:Ignoring the fine structure splitting, hyperfine structure splitting, etc., such that energy
levels depend only on the principal quantum number n, how many distinct states of
singly-ionized helium (Z = 2) have energy E= -13.6 eV? Write out all the quantum
numbers (n, l, me, ms) describing each distinct state. (Recall that the ground state
energy of hydrogen is E₁ = -13.6 eV, and singly-ionized helium may be treated as a
hydrogen-like atom.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images
