In a first order chemical reaction with no backorder reaction the concentration of the reactant is governed by dc kC ; where C is the dt concentration of the single reactant, t is the time and k is the function of temperature called the rate ĉonstant. Solve the equation to find C as function of t.
In a first order chemical reaction with no backorder reaction the concentration of the reactant is governed by dc kC ; where C is the dt concentration of the single reactant, t is the time and k is the function of temperature called the rate ĉonstant. Solve the equation to find C as function of t.
Algebra & Trigonometry with Analytic Geometry
13th Edition
ISBN:9781133382119
Author:Swokowski
Publisher:Swokowski
Chapter5: Inverse, Exponential, And Logarithmic Functions
Section: Chapter Questions
Problem 18T
Related questions
Question
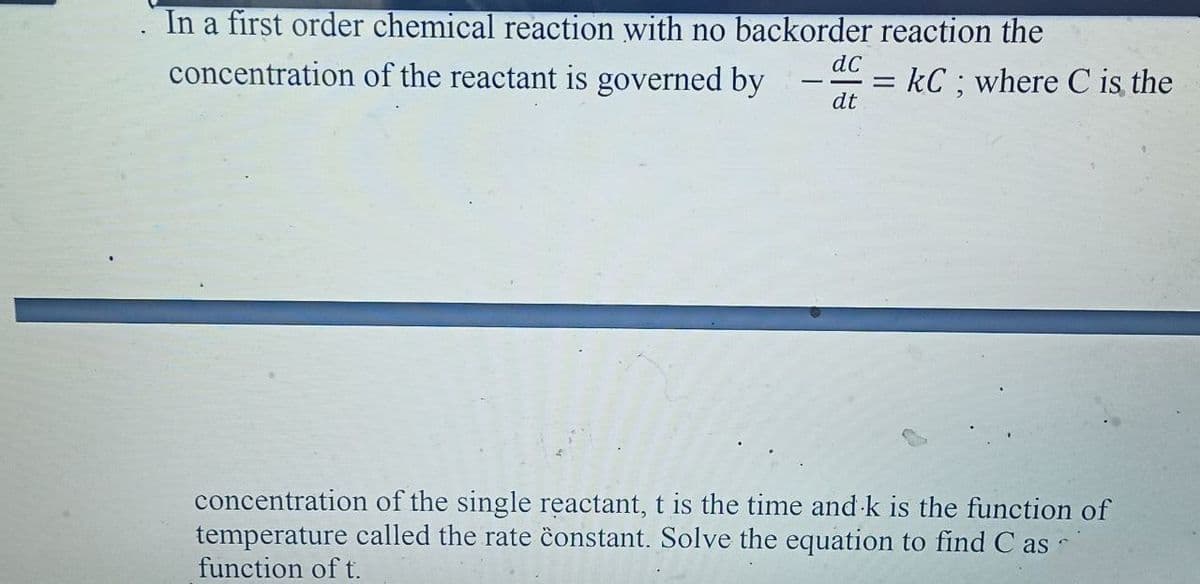
Transcribed Image Text:In a first order chemical reaction with no backorder reaction the
concentration of the reactant is governed by
dC
= kC; where C is the
%D
dt
concentration of the single reactant, t is the time and k is the function of
temperature called the rate ĉonstant. Solve the equation to find C as
function of t.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Linear Algebra: A Modern Introduction
Algebra
ISBN:
9781285463247
Author:
David Poole
Publisher:
Cengage Learning