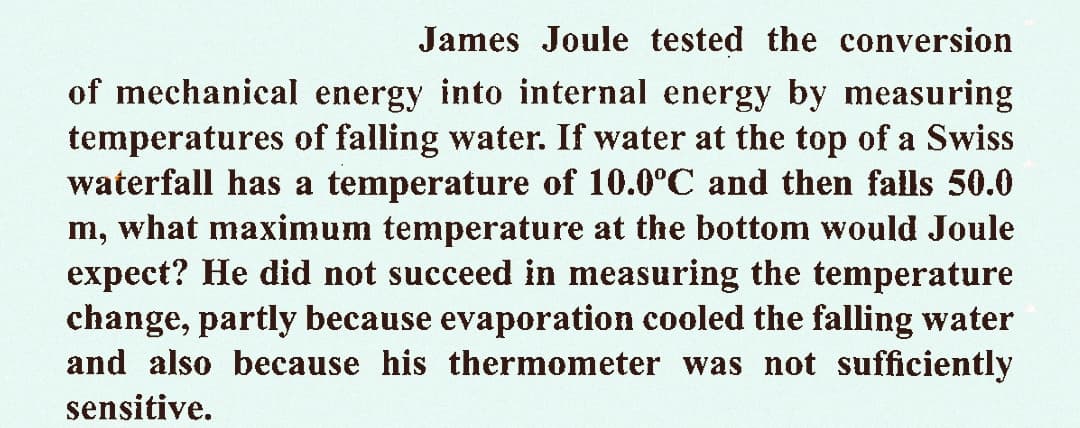
James Joule tested the conversion of mechanical energy into internal energy by measuring temperatures of falling water. If water at the top of a Swiss waterfall has a temperature of 10.0°C and then falls 50.0 m, what maximum temperature at the bottom would Joule expect? He did not succeed in measuring the temperature change, partly because evaporation cooled the falling water and also because his thermometer was not sufficiently sensitive.
James Joule tested the conversion of mechanical energy into internal energy by measuring temperatures of falling water. If water at the top of a Swiss waterfall has a temperature of 10.0°C and then falls 50.0 m, what maximum temperature at the bottom would Joule expect? He did not succeed in measuring the temperature change, partly because evaporation cooled the falling water and also because his thermometer was not sufficiently sensitive.
Related questions
Question
Transcribed Image Text:James Joule tested the conversion
of mechanical energy into internal energy by measuring
temperatures of falling water. If water at the top of a Swiss
waterfall has a temperature of 10.0°C and then falls 50.0
m, what maximum temperature at the bottom would Joule
expect? He did not succeed in measuring the temperature
change, partly because evaporation cooled the falling water
and also because his thermometer was not sufficiently
sensitive.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps
