Jhe probability.distribution for bromine gas is shown in the diagram below. Determine the temperature of the gas. Take the molar mass of bromine to be 160 g/mol. "Whät is the significance of the peak value of the speed in the probability distribution curve? How is the temperature of the gas related to the most probable speed? K V (m/s) 480 960 1440 1920 2400
Jhe probability.distribution for bromine gas is shown in the diagram below. Determine the temperature of the gas. Take the molar mass of bromine to be 160 g/mol. "Whät is the significance of the peak value of the speed in the probability distribution curve? How is the temperature of the gas related to the most probable speed? K V (m/s) 480 960 1440 1920 2400
Related questions
Question
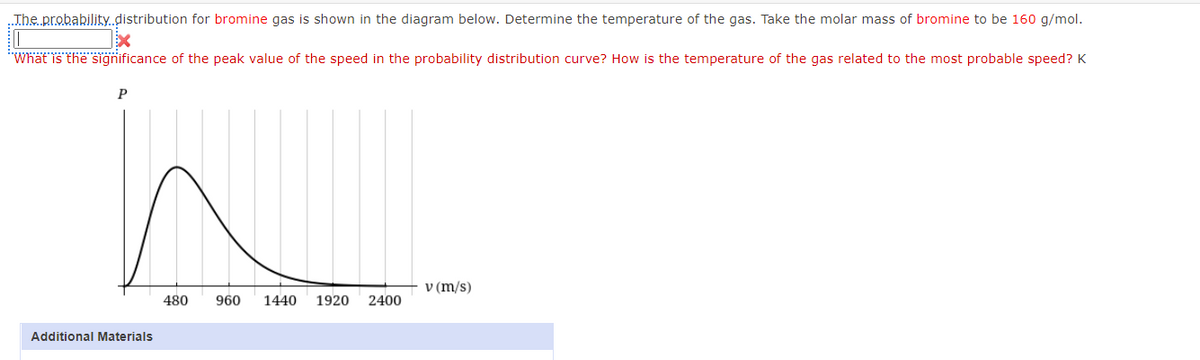
Transcribed Image Text:.The probability.distribution for bromine gas is shown in the diagram below. Determine the temperature of the gas. Take the molar mass of bromine to be 160 g/mol.
"Whät is the sigğnificance of the peak value of the speed in the probability distribution curve? How is the temperature of the gas related to the most probable speed? K
P
v (m/s)
480
960
1440
1920
2400
Additional Materials
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images
