(Kittel 6.11) Convective isentropic equilibrium of the atmosphere. The lower 10- 15 km of the atmosphere - the troposphere -is often in a convective steady state at constant entropy, not constant temperature. In such equilibrium pV is independent of altitude, where y = C₁/C₁. Use the condition of mechanical equilibrium in a uniform gravitational field to: (a) Show that dT/dz = constant, where z is the altitude. This quantity, important in meteorology, is called the dry adiabatic lapse rate. (Do not use the barometric pressure relation that was derived in Chapter 5 for an isothermal atmosphere.) (b) Estimate dT/dz, in degrees Celsius per km. Take y = 7/5. (c) Show that p~ p', where p is the mass density. If the actual temperature gradient is greater than the isentropic gradient, the atmosphere may be unstable with respect to convection.
(Kittel 6.11) Convective isentropic equilibrium of the atmosphere. The lower 10- 15 km of the atmosphere - the troposphere -is often in a convective steady state at constant entropy, not constant temperature. In such equilibrium pV is independent of altitude, where y = C₁/C₁. Use the condition of mechanical equilibrium in a uniform gravitational field to: (a) Show that dT/dz = constant, where z is the altitude. This quantity, important in meteorology, is called the dry adiabatic lapse rate. (Do not use the barometric pressure relation that was derived in Chapter 5 for an isothermal atmosphere.) (b) Estimate dT/dz, in degrees Celsius per km. Take y = 7/5. (c) Show that p~ p', where p is the mass density. If the actual temperature gradient is greater than the isentropic gradient, the atmosphere may be unstable with respect to convection.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
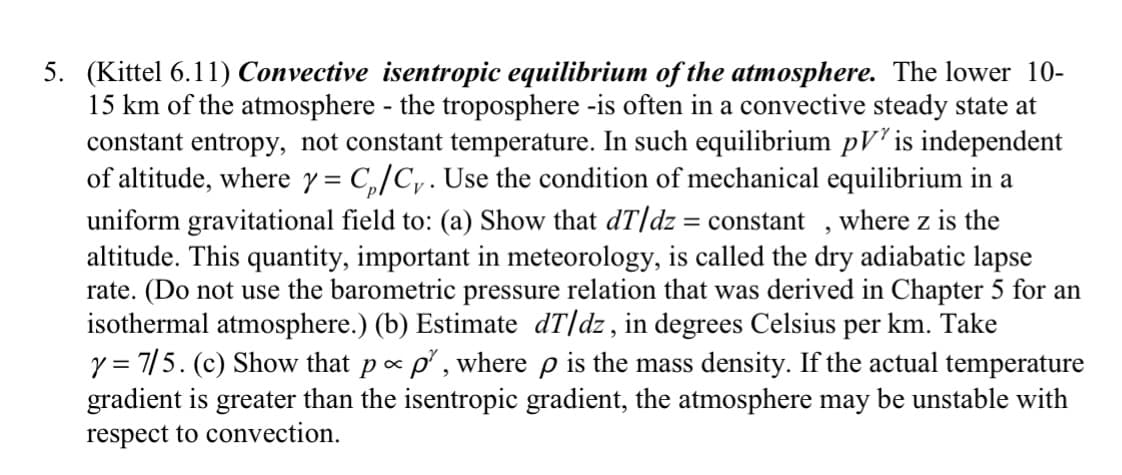
Transcribed Image Text:5. (Kittel 6.11) Convective isentropic equilibrium of the atmosphere. The lower 10-
15 km of the atmosphere - the troposphere -is often in a convective steady state at
constant entropy, not constant temperature. In such equilibrium pV² is independent
of altitude, where y = C₁/C. Use the condition of mechanical equilibrium in a
uniform gravitational field to: (a) Show that dT/dz = constant, where z is the
altitude. This quantity, important in meteorology, is called the dry adiabatic lapse
rate. (Do not use the barometric pressure relation that was derived in Chapter 5 for an
isothermal atmosphere.) (b) Estimate dT/dz, in degrees Celsius per km. Take
y = 7/5. (c) Show that p- p², where p is the mass density. If the actual temperature
gradient is greater than the isentropic gradient, the atmosphere may be unstable with
respect to convection.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The