Look at the model of a molecule below: Which statements are true about this model? (choose all of the correct answers) This molecule is an example of a single element. There are 9 different types of atoms in this molecule. This substance contains 2 atoms of carbon. D If one atom of hydrogen were removed, this would remain the same substance E The chemical formula for this molecule is C>HO This molecule contains three different tvpes of elements.
Look at the model of a molecule below: Which statements are true about this model? (choose all of the correct answers) This molecule is an example of a single element. There are 9 different types of atoms in this molecule. This substance contains 2 atoms of carbon. D If one atom of hydrogen were removed, this would remain the same substance E The chemical formula for this molecule is C>HO This molecule contains three different tvpes of elements.
Related questions
Question
Transcribed Image Text:testing.iluminateed.com/assessment/6155f25ec91909.
Question 3
II Pause
Q Zoom
ABC
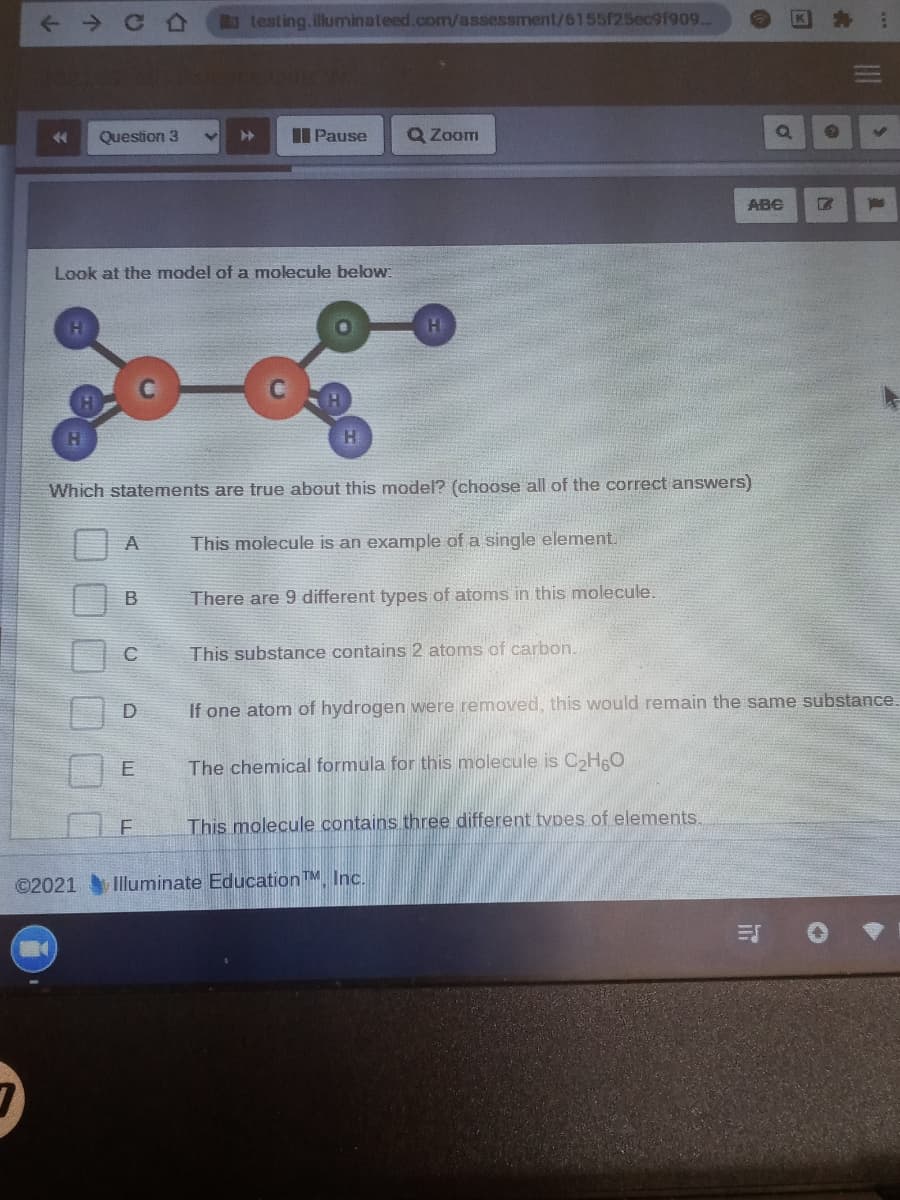
Look at the model of a molecule below:
Which statements are true about this model? (choose all of the correct answers)
A
This molecule is an example of a single element.
There are 9 different types of atoms in this molecule.
This substance contains 2 atoms of carbon.
If one atom of hydrogen were removed, this would remain the same substance.
E
The chemical formula for this molecule is CHO
This molecule contains three different tvpes of elements.
©2021 Iluminate Education M, Inc.
LL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images
