Mass Measurements (proce
Related questions
Question
Transcribed Image Text:1013 %
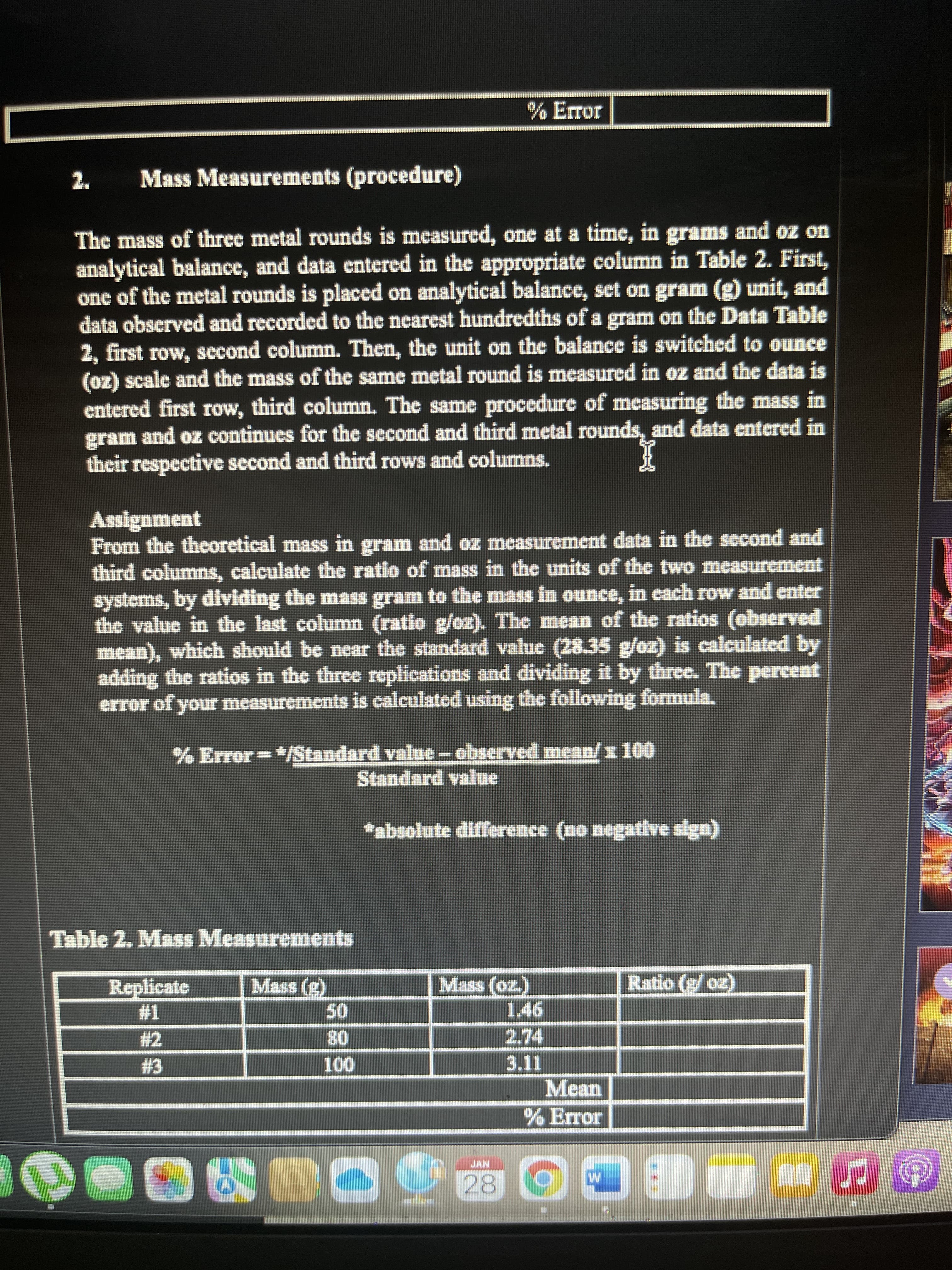
Mass Measurements (procedure)
The mass of three metal rounds is measured, one at a time, in grams and oz on
analytical balance, and data entered in the appropriate column in Table 2. First,
one of the metal rounds is placed on analytical balance, set on gram (g) unit, and
data obscrved and recorded to the nearest hundredths of a gram on the Data Table
2, first row, second column. Then, the unit on the balance is switched to ounce
(oz) scale and the mass of the same metal round is measured in oz and the data is
entered first row, third column. The same procedure of measuring the mass in
gram and oz continues for the second and third metal rounds, and data entered in
their respective second and third rows and columns.
Assignment
From the theoretical mass in gram and oz measurement data in the second and
third columns, calculate the ratio of mass in the units of the two measurement
systems, by dividing the mass gram to the mass in ounce, in each row and enter
the value in the last column (ratio g/oz). The mean of the ratios (observed
mean), which should be near the standard value (28.35 g/oz) is calculated by
adding the ratios in the three replications and dividing it by three. The percent
error of your measurements is calculated using the following formula.
% Error=*/Standard value-observed mean/x 100
Standard value
*absolute difference (no negative sign)
Table 2. Mass Measurements
Replicate
Mass (g)
Mass (oz.)
(20 /3) o
T#
# 2
1.46
2.74
08
3.11
000
Mean
% Error
马a0aD
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps
