Match the definition and example to the type of reaction. < Zn and Cu2+ react. Zn loses 2 electrons to become Zn2+ and Cu gains 2 electrons to become Cu. CHCOCH,CHICH, +16,00--0H + COCHICH 10 Butylan Wi And B
Match the definition and example to the type of reaction. < Zn and Cu2+ react. Zn loses 2 electrons to become Zn2+ and Cu gains 2 electrons to become Cu. CHCOCH,CHICH, +16,00--0H + COCHICH 10 Butylan Wi And B
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:Elaine N. Marieb, Katja N. Hoehn
Chapter1: The Human Body: An Orientation
Section: Chapter Questions
Problem 1RQ: The correct sequence of levels forming the structural hierarchy is A. (a) organ, organ system,...
Related questions
Question
Question in Image, all one part. Thanks you!!!
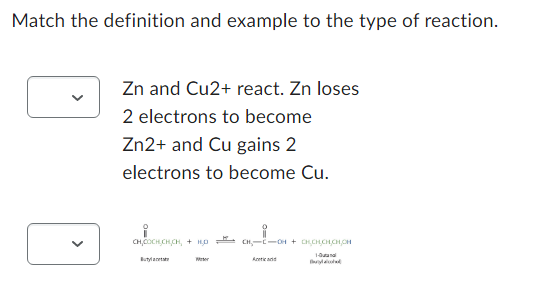
Transcribed Image Text:Match the definition and example to the type of reaction.
<
Zn and Cu2+ react. Zn loses
2 electrons to become
Zn2+ and Cu gains 2
electrons to become Cu.
CHO CHCHCH, 4 HỌ
Buty
Wer
CH₂-C-OH + CHCH,CH,CH,CH
1-Butanol
And

Transcribed Image Text:<
HCI + NaOH --> NaCl +
H2O
These reactions consist of
the removal of an -OH and
an -H from two reactant
molecules.
This type of reaction
involves something with a
pH level greater than 7
reacting with a substance
that has a pH level less than
7.
Breaking molecules apart
by reaction with water.
glucose + glucose -->
maltose + water
One thing is oxidized and
another thing is reduced in
this type of reaction.
1. Hydrolysis reaction.
2. Dehydration reaction
3. Redox reaction
4. Acid-base reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:
9780134580999
Author:
Elaine N. Marieb, Katja N. Hoehn
Publisher:
PEARSON

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Anatomy & Physiology
Biology
ISBN:
9781259398629
Author:
McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:
Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:
9780815344322
Author:
Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:
W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:
9781260159363
Author:
Martin, Terry R., Prentice-craver, Cynthia
Publisher:
McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:
9781260231700
Author:
Sylvia S. Mader, Michael Windelspecht
Publisher:
McGraw Hill Education