of hydrogen ions measured in moles per liter. A particular solution has a concentration of hydrogen ions of 2.88 x 10 moles per liter. Compute the pH level for this solution. Give at least 3 decimal places. pH level =
of hydrogen ions measured in moles per liter. A particular solution has a concentration of hydrogen ions of 2.88 x 10 moles per liter. Compute the pH level for this solution. Give at least 3 decimal places. pH level =
Algebra & Trigonometry with Analytic Geometry
13th Edition
ISBN:9781133382119
Author:Swokowski
Publisher:Swokowski
Chapter5: Inverse, Exponential, And Logarithmic Functions
Section5.6: Exponential And Logarithmic Equations
Problem 52E
Related questions
Question
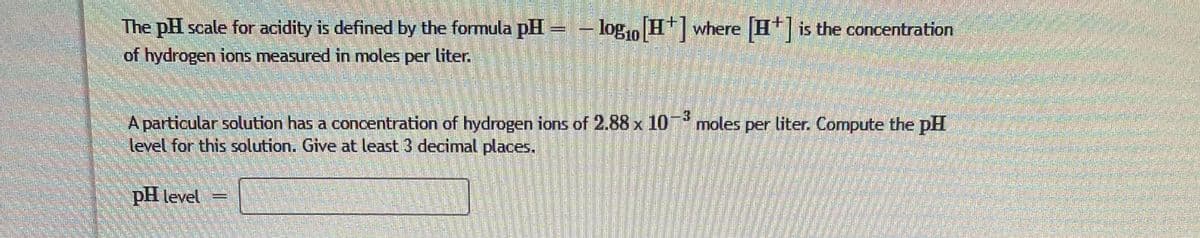
Transcribed Image Text:- log, H where His the concentration
The pH scale for acidity is defined by the formula pH-
of hydrogen ions measured in moles per liter.
3
A particular solution has a concentration of hydrogen ions of 2.88 x 10* moles per liter. Compute the pH
level for this solution. Give at least 3 decimal places.
pH level =
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Algebra and Trigonometry (MindTap Course List)
Algebra
ISBN:
9781305071742
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Algebra and Trigonometry (MindTap Course List)
Algebra
ISBN:
9781305071742
Author:
James Stewart, Lothar Redlin, Saleem Watson
Publisher:
Cengage Learning

Functions and Change: A Modeling Approach to Coll…
Algebra
ISBN:
9781337111348
Author:
Bruce Crauder, Benny Evans, Alan Noell
Publisher:
Cengage Learning
