ork concentration profile c(r) of molecules in a centrifuge, which is Nelson's Eq. A sample is spinning at an angular velocity w (in radians/sec). The net mass or effective mass (as in our earlier lemonade problem) is mnet. From the view of molecules spinning at a distance r from the axis of spinning, there is an outwards force (the centrifugal force) f(r) = mnetw^2r (the same thing as mnetv2/r). You'll need to remember how this force gives you a potential energy that is seen by the molecules. It's this potential energy that should ho used in a Boltzmann distribution
ork concentration profile c(r) of molecules in a centrifuge, which is Nelson's Eq. A sample is spinning at an angular velocity w (in radians/sec). The net mass or effective mass (as in our earlier lemonade problem) is mnet. From the view of molecules spinning at a distance r from the axis of spinning, there is an outwards force (the centrifugal force) f(r) = mnetw^2r (the same thing as mnetv2/r). You'll need to remember how this force gives you a potential energy that is seen by the molecules. It's this potential energy that should ho used in a Boltzmann distribution
Related questions
Question
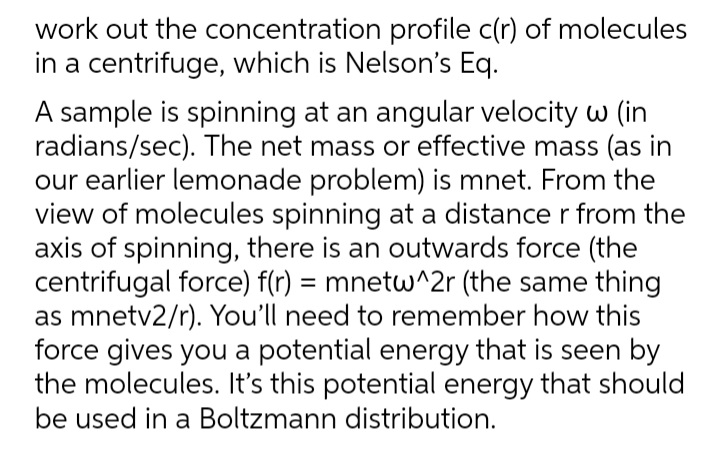
Transcribed Image Text:work out the concentration profile c(r) of molecules
in a centrifuge, which is Nelson's Eq.
A sample is spinning at an angular velocity w (in
radians/sec). The net mass or effective mass (as in
our earlier lemonade problem) is mnet. From the
view of molecules spinning at a distance r from the
axis of spinning, there is an outwards force (the
centrifugal force) f(r) = mnetw^2r (the same thing
as mnetv2/r). You'll need to remember how this
force gives you a potential energy that is seen by
the molecules. It's this potential energy that should
be used in a Boltzmann distribution.
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps
