P5. Supercooled water II In an isolated container, which has cooling tubes built in the walls, an amount of m = 18 kg clean water is very carefully cooled down to the temperature of ti = -9 °C. After this a small ice crystal of negligible mass is thrown into the water, which starts the freezing of the supercooled water. Determine the amount of ice produced!
P5. Supercooled water II In an isolated container, which has cooling tubes built in the walls, an amount of m = 18 kg clean water is very carefully cooled down to the temperature of ti = -9 °C. After this a small ice crystal of negligible mass is thrown into the water, which starts the freezing of the supercooled water. Determine the amount of ice produced!
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
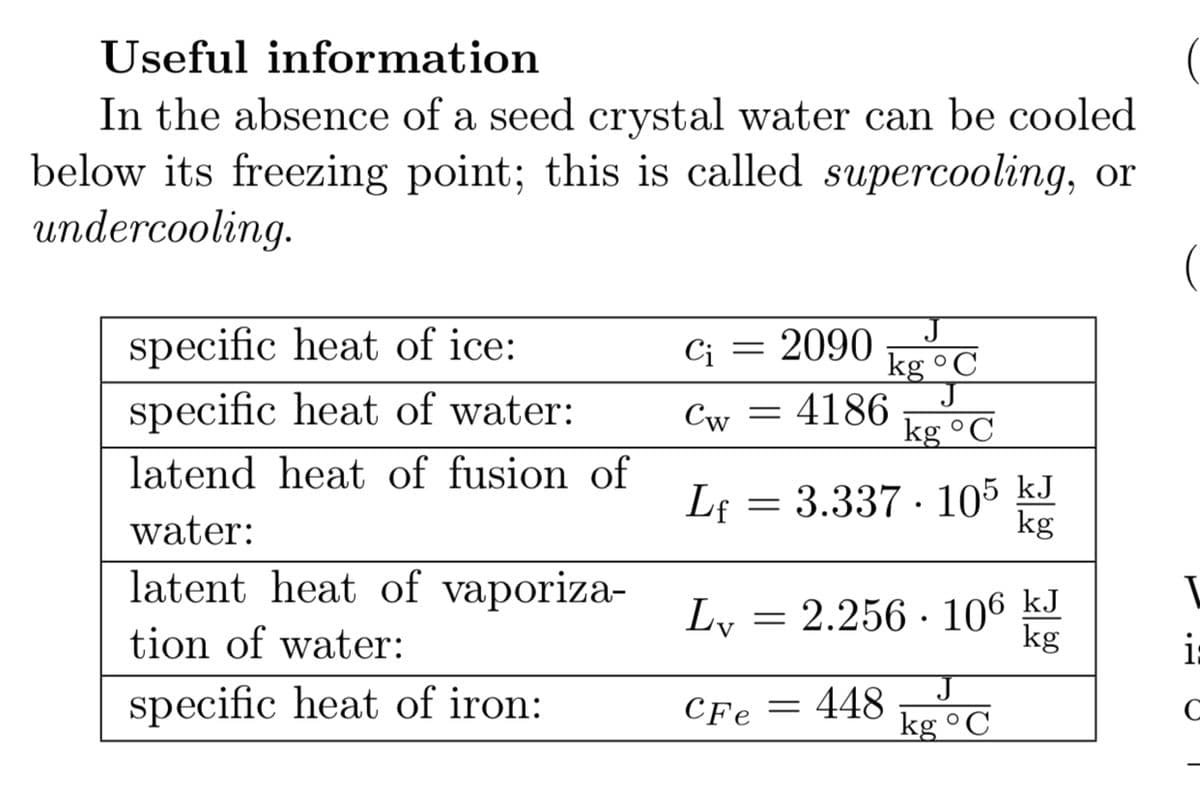
Transcribed Image Text:Useful information
In the absence of a seed crystal water can be cooled
below its freezing point; this is called supercooling, or
undercooling.
specific heat of ice:
2090
kg °C
Ci
specific heat of water:
Cw = 4186
kg °C
latend heat of fusion of
Lf = 3.337 · 105 kJ
water:
latent heat of vaporiza-
Ly =
2.256 · 106 kJ
kg
tion of water:
i:
specific heat of iron:
CFe = 448
kg °C
C

Transcribed Image Text:P4. Supercooled water I
When 1 kg of water freezes at 0°C, then 333.7 kJ heat
is released. Determine the heat released while 1 kg
of supercooled water freezes at constant temperature
-10°C. (Neglect the difference between the densities
of water and ice.)
|
P5. Supercooled water II
In an isolated container, which has cooling tubes built
in the walls, an amount of m = 18 kg clean water
is very carefully cooled down to the temperature of
ti = -9 °C. After this a small ice crystal of negligible
mass is thrown into the water, which starts the freezing
of the supercooled water. Determine the amount of ice
produced!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The