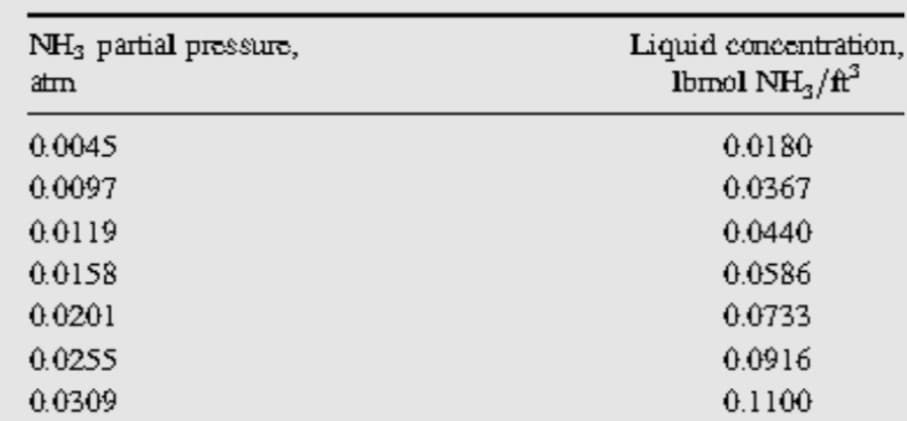
pilot scale absorber column. In a laboratory test under atmospheric conditions (70 ° F and 1 atm). The absorber was installed in such a way as to measure the partial pressure of each component at various locations in the column. It was found that at a distance of 5 feet high (mid-column), the liquid contained 0.4% by weight of ammonia, while the pressure of the ammonia in the gas (put in contact with the mentioned liquid) was 10 mm Hg. Estimate the interfacial concentrations of the liquid and gas phases. Based on previous experiments, the ratio of the liquid mass transfer coefficients kL / kG turned out to be 1.0 atm / (lb mol / ft3). The solubility data fo
A mixture of air and ammonia is being treated, with a stream of water (used as a solvent), in a 10 foot high pilot scale absorber column. In a laboratory test under atmospheric conditions (70 ° F and 1 atm). The absorber was installed in such a way as to measure the partial pressure of each component at various locations in the column. It was found that at a distance of 5 feet high (mid-column), the liquid contained 0.4% by weight of ammonia, while the pressure of the ammonia in the gas (put in contact with the mentioned liquid) was 10 mm Hg. Estimate the interfacial concentrations of the liquid and gas phases.
Based on previous experiments, the ratio of the liquid
kL / kG turned out to be 1.0 atm / (lb mol / ft3). The solubility data for this system is presented below:
Step by step
Solved in 2 steps with 1 images









