Problem 1: Rubber bands and Universality Polymers, like rubber, are made of very long molecules, usually tangled up in a configuration with lots of entropy. As a crude model of a rubber band, consider a chain of N links, each of length e (see below). Imaging that each link has only two possible states, pointing either left or right. The total length L of the rubber band is the net displacement from the beginning of the first link to the end of the last link. N links a) Find an expression for the entropy of this system in terms of N and Ng, the number of links pointing to the right. b) Write down a formula for L in terms of N and Ng. c) For a one-dimensional system such as this, the length L is analogous to the volume V of a three-dimensional system. Similarly, the pressure P is replaced by the tension force F. Taking F to be positive when the rubber band is pulling inward, write down and explain the appropriate thermodynamic identity for this system. d) Using the thermodynamic identity, you can now express the tension force F in terms of a partial derivative of the entropy. From this expression, compute the tension in terms of L, T, N, and l. e) Show that when L « Ne, then tension force is directly proportional to L (Hooke's law). f) Discuss the dependence of the tension force on temperature. If you increase the temperature of a rubber band, does it tend to expand or contract? Does this behavior make sense? g) Suppose that you hold a relaxed rubber band in both hands and suddenly stretch it. Would you expect its temperature to increase or decrease? Test your prediction with a real rubber band (preferably a heavy one with lots of stretch), using your lips or forehead as a thermometer.
Problem 1: Rubber bands and Universality Polymers, like rubber, are made of very long molecules, usually tangled up in a configuration with lots of entropy. As a crude model of a rubber band, consider a chain of N links, each of length e (see below). Imaging that each link has only two possible states, pointing either left or right. The total length L of the rubber band is the net displacement from the beginning of the first link to the end of the last link. N links a) Find an expression for the entropy of this system in terms of N and Ng, the number of links pointing to the right. b) Write down a formula for L in terms of N and Ng. c) For a one-dimensional system such as this, the length L is analogous to the volume V of a three-dimensional system. Similarly, the pressure P is replaced by the tension force F. Taking F to be positive when the rubber band is pulling inward, write down and explain the appropriate thermodynamic identity for this system. d) Using the thermodynamic identity, you can now express the tension force F in terms of a partial derivative of the entropy. From this expression, compute the tension in terms of L, T, N, and l. e) Show that when L « Ne, then tension force is directly proportional to L (Hooke's law). f) Discuss the dependence of the tension force on temperature. If you increase the temperature of a rubber band, does it tend to expand or contract? Does this behavior make sense? g) Suppose that you hold a relaxed rubber band in both hands and suddenly stretch it. Would you expect its temperature to increase or decrease? Test your prediction with a real rubber band (preferably a heavy one with lots of stretch), using your lips or forehead as a thermometer.
Related questions
Question
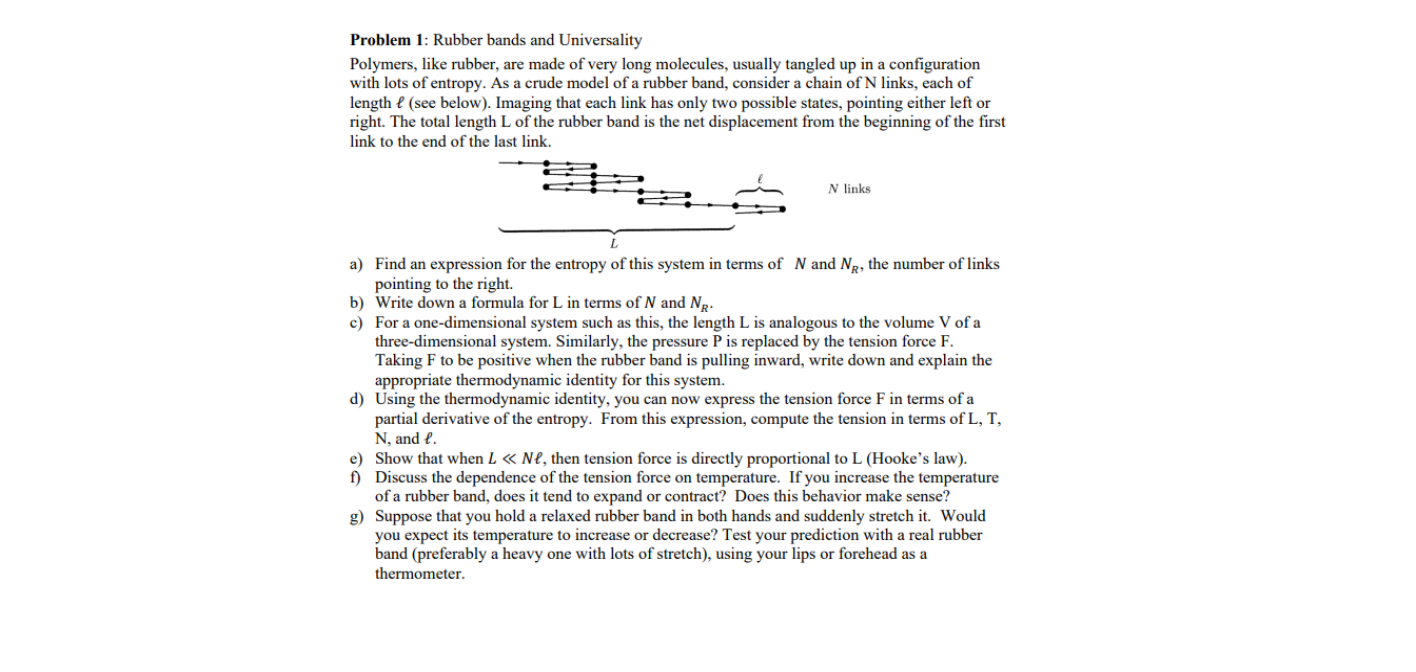
Transcribed Image Text:Problem 1: Rubber bands and Universality
Polymers, like rubber, are made of very long molecules, usually tangled up in a configuration
with lots of entropy. As a crude model of a rubber band, consider a chain of N links, each of
length e (see below). Imaging that each link has only two possible states, pointing either left or
right. The total length L of the rubber band is the net displacement from the beginning of the first
link to the end of the last link.
N links
a) Find an expression for the entropy of this system in terms of N and Ng, the number of links
pointing to the right.
b) Write down a formula for L in terms of N and Ng.
c) For a one-dimensional system such as this, the length L is analogous to the volume V of a
three-dimensional system. Similarly, the pressure P is replaced by the tension force F.
Taking F to be positive when the rubber band is pulling inward, write down and explain the
appropriate thermodynamic identity for this system.
d) Using the thermodynamic identity, you can now express the tension force F in terms of a
partial derivative of the entropy. From this expression, compute the tension in terms of L, T,
N, and l.
e) Show that when L « Ne, then tension force is directly proportional to L (Hooke's law).
f) Discuss the dependence of the tension force on temperature. If you increase the temperature
of a rubber band, does it tend to expand or contract? Does this behavior make sense?
g) Suppose that you hold a relaxed rubber band in both hands and suddenly stretch it. Would
you expect its temperature to increase or decrease? Test your prediction with a real rubber
band (preferably a heavy one with lots of stretch), using your lips or forehead as a
thermometer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 6 images
