Problem 19.31 Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9 L to 9.3 L, where the temperature reaches its original value. (See figure(Figure 1)). Part A Calculate the total work done by the gas in the process. Express your answer using two significant figures. ? W = Submit Request Answer Part B Figure 1 of 1 Calculate the change in internal energy of the gas in the process. VO AEO ? 2.2 atm AEint = J b. 1.4 atm Submit Request Answer Part C 5.9 L 9.3 L V Calculate the total heat flow into or out of the gas
Problem 19.31 Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9 L to 9.3 L, where the temperature reaches its original value. (See figure(Figure 1)). Part A Calculate the total work done by the gas in the process. Express your answer using two significant figures. ? W = Submit Request Answer Part B Figure 1 of 1 Calculate the change in internal energy of the gas in the process. VO AEO ? 2.2 atm AEint = J b. 1.4 atm Submit Request Answer Part C 5.9 L 9.3 L V Calculate the total heat flow into or out of the gas
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter2: Matter And Energy
Section: Chapter Questions
Problem 30RQ: A gas is compressed inside a compressor's cylinder. When the piston is at its bottom dead center,...
Related questions
Question
100%
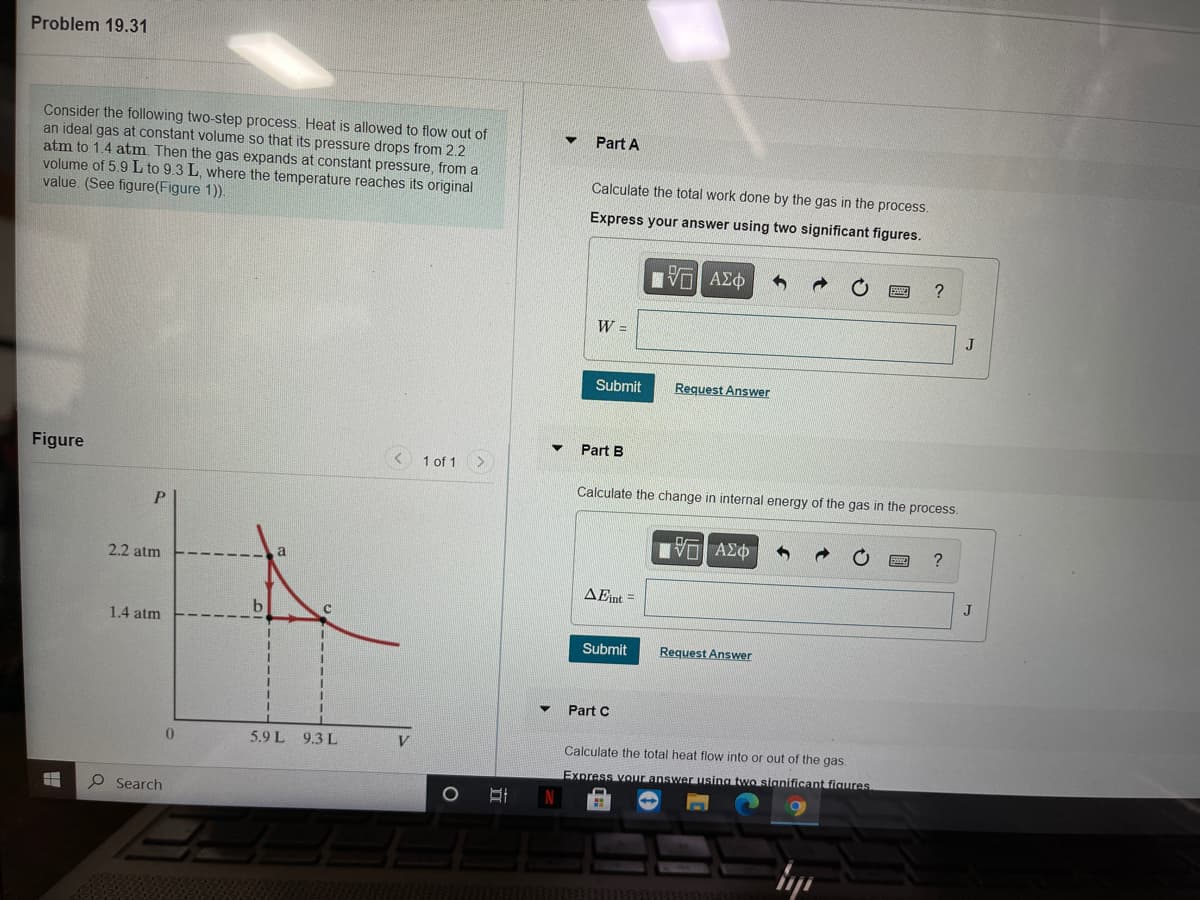
Transcribed Image Text:Problem 19.31
Consider the following two-step process. Heat is allowed to flow out of
an ideal gas at constant volume so that its pressure drops from 2.2
atm to 1.4 atm. Then the gas expands at constant pressure, from a
volume of 5.9 L to 9.3 L, where the temperature reaches its original
value. (See figure(Figure 1)).
Part A
Calculate the total work done by the gas in the process.
Express your answer using two significant figures.
AEO
?
W =
Submit
Request Answer
Part B
Figure
1 of 1
Calculate the change in internal energy of the gas in the process.
P.
μνα ΑΣφ
?
2.2 atm
AEint =
J
1.4 atm
Submit
Request Answer
Part C
5.9 L 9.3 L
Calculate the total heat flow into or out of the gas.
Express vour answer using two sianificant figures
P Search
立
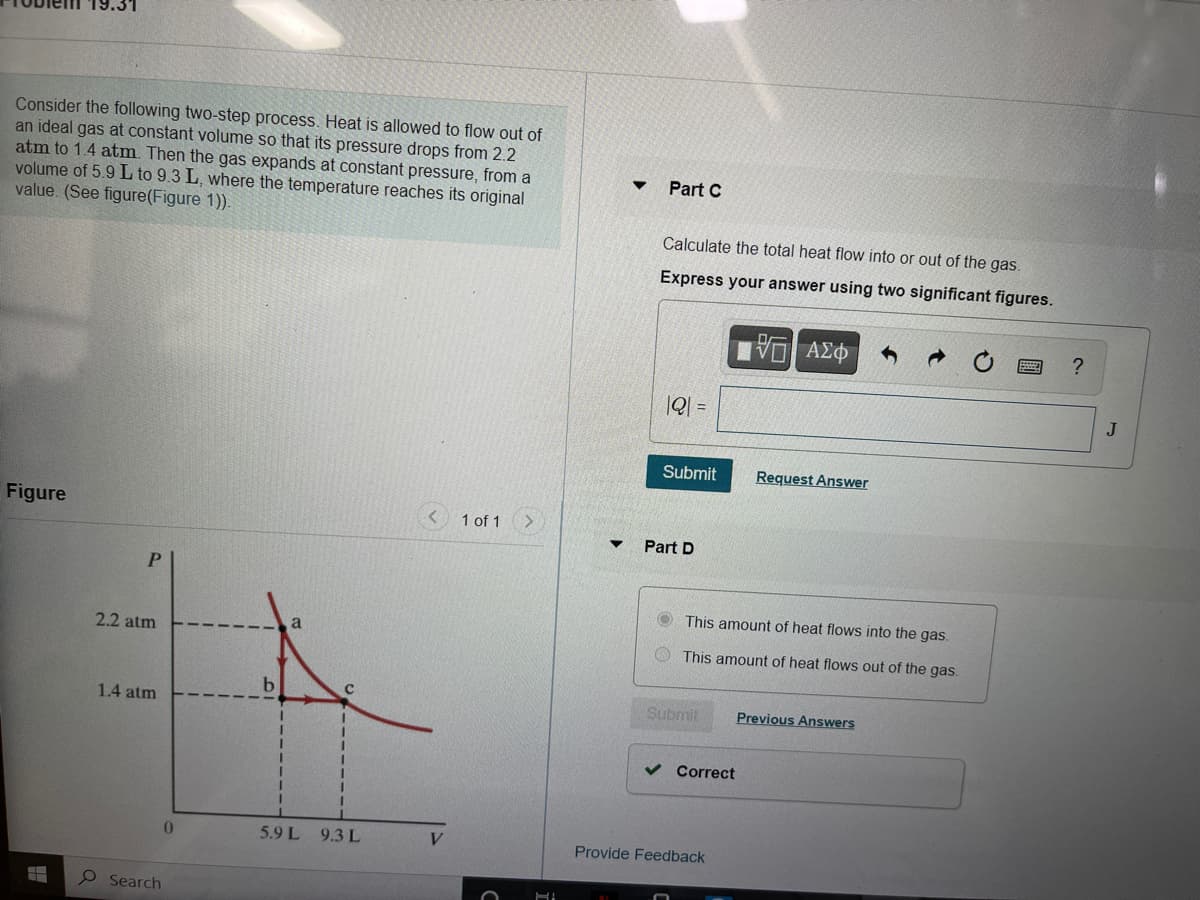
Transcribed Image Text:19.31
Consider the following two-step process. Heat is allowed to flow out of
an ideal gas at constant volume so that its pressure drops from 2.2
atm to 1.4 atm. Then the gas expands at constant pressure, from a
volume of 5.9 L to 9.3 L, where the temperature reaches its original
value. (See figure(Figure 1)).
Part C
Calculate the total heat flow into or out of the gas.
Express your answer using two significant figures.
Q| =
J
Submit
Request Answer
Figure
1 of 1
Part D
O This amount of heat flows into the gas.
2.2 atm
This amount of heat flows out of the gas.
b.
Submit
Previous Answers
1.4 atm
Correct
5.9 L 9.3L
V
Provide Feedback
Search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning