Q1) An inventor wants to build a hydrogen manometer based on the dependence of the output voltage of a fuel cell on the pressure of the reactants. Take a H,/O, fuel cell operating at 298 K. Assume that it produces water vapor and that the open circuit voltage is that of an ideal cell. The oxygen pressure is maintained at a constant 0.103 MPa while the hydrogen pressure, pH2 , measured. What is the output voltage when pu2 are 0.103 and 1.1 MPa? the free energy change is -230 MJ/kmole of H,O at RTP. Use Gas constant R: 8314 J mol" K is the quantity to be
Q1) An inventor wants to build a hydrogen manometer based on the dependence of the output voltage of a fuel cell on the pressure of the reactants. Take a H,/O, fuel cell operating at 298 K. Assume that it produces water vapor and that the open circuit voltage is that of an ideal cell. The oxygen pressure is maintained at a constant 0.103 MPa while the hydrogen pressure, pH2 , measured. What is the output voltage when pu2 are 0.103 and 1.1 MPa? the free energy change is -230 MJ/kmole of H,O at RTP. Use Gas constant R: 8314 J mol" K is the quantity to be
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
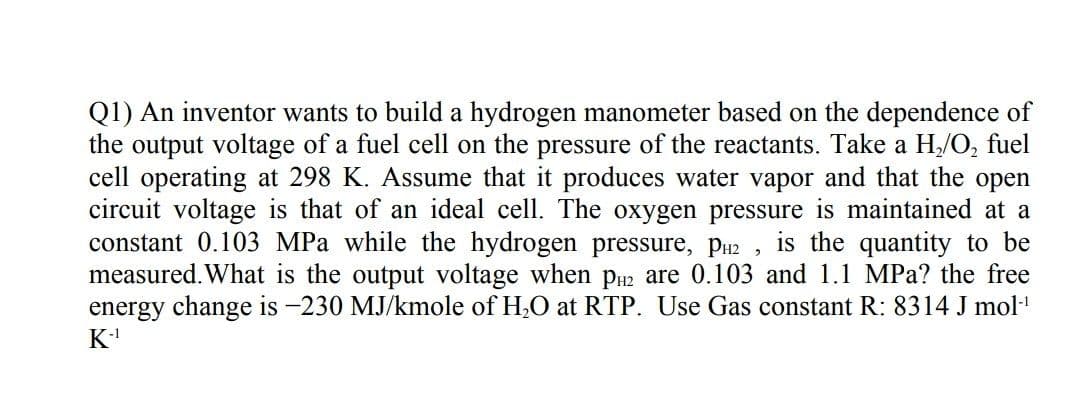
Transcribed Image Text:Q1) An inventor wants to build a hydrogen manometer based on the dependence of
the output voltage of a fuel cell on the pressure of the reactants. Take a H/O, fuel
cell operating at 298 K. Assume that it produces water vapor and that the open
circuit voltage is that of an ideal cell. The oxygen pressure is maintained at a
constant 0.103 MPa while the hydrogen pressure, PH2 ,
measured. What is the output voltage when pu2 are 0.103 and 1.1 MPa? the free
energy change is -230 MJ/kmole of H,O at RTP. Use Gas constant R: 8314 J mol
K
is the quantity to be
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The